 Your new post is loading...
 Your new post is loading...
Chimeric Antigen Receptor (CAR) refers to a novel technology that leverages the immune system in a rapid, potent and direct manner against cancer. CARs are engineered receptors that can target surface molecules of interest expressed on tumor cells. CARs typically engage the target antigen via a single-chain variable fragment (scFv) derived from a monoclonal antibody. Beyond the scFv, the hinge, transmembrane and intracellular signaling domains of CAR make important contributions to the interaction with antigen, assembly of the immunologic synapse, and association of the CAR with other proteins necessary to transduce a robust activation signal.
Formula’s technology is distinctive in that it uses a different kind of immune effector cell for CAR directed therapy, namely Cytokine Induced Killer (C.I.K.) cells (rather than T cells for CAR-T). C.I.K. cells are lymphocytes that also have Natural Killer (NK) cell-like properties, despite the fact that 95% of the C.I.K. cell population has the CD3+, T cell phenotype. The NK-like properties involve immune-cell activation mechanisms different from T cells, in that the NK-like anti-tumor response is non-MHC restricted. Because of these additional features, C.I.K. cells may therefore have a broader therapeutic impact.
Via Krishan Maggon
The MaxCyte GT® Flow Transfection System is a universal platform technology for rapid, automated loading of CAR (chimeric antigen receptor) mRNA into peripheral blood mononuclear cells using closed-system aseptic processing.
Via Krishan Maggon
Highlights • T cells undergo metabolic remodeling to support their function. • Metabolic pathways impact on T cell differentiation decisions and function in the periphery. • Manipulating metabolic microenvironments may enhance T cell function in cancer. • Metabolic pathways could be targeted for the treatment of human disease. In the past several years a wealth of evidence has emerged illustrating how metabolism supports many aspects of T cell biology, as well as how metabolic changes drive T cell differentiation and fate. We outline developing principles in the regulation of T cell metabolism, and discuss how these processes are affected in settings of inflammation and cancer. In this context we discuss how metabolic pathways might be manipulated for the treatment of human disease, including how metabolism may be targeted to prevent T cell dysfunction in inhospitable microenvironments, to generate more effective adoptive cellular immunotherapies in cancer, and to direct T cell differentiation and function towards non-pathogenic phenotypes in settings of autoimmunity.
Via Krishan Maggon
RT @CeriFielding: Natural killer cells #Immunotherapy @NatureMedicine http://t.co/R37PGlyaLj" ; Cancer immunotherapy—which trains the body's own immune system to fight tumors—has made medical headlines in the last few years, with analysts projecting that it could give rise to treatments worth $35 billion a year over the next decade. For the most part, cancer immunotherapy has relied on the power of T cells. Now, another class of immune cells—known as natural killer (NK) cells—that can function to kill cancer is nearing its big break. NK cells may hold the potential to kill off cancer cells without damaging healthy tissues or risking the T cell–driven inflammatory cytokine storm that can accompany other immunotherapies. But a complicated assortment of protein receptors that control their function makes NK cells unwieldy and unpredictable. Dr. Porter, UPenn spoke Sunday at the 35th Annual Conference on Clinical Hematology & Oncology, held in La Jolla by Scripps Health.
Via Krishan Maggon
Atara Biotherapeutics, Inc. (Nasdaq:ATRA) today announced that its collaborative partner, Memorial Sloan Kettering Cancer Center (MSK) has received breakthrough therapy designation from the U.S. Food and Drug Administration (FDA) for Atara's optioned cytotoxic T lymphocytes activated against Epstein-Barr Virus (EBV-CTL) in the treatment of patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), a type of malignancy occurring after allogeneic hematopoietic cell transplantation (HCT). Allogeneic HCT is also commonly called a bone marrow transplant. - The T-cell collaboration with MSK consists of three types of CTLs, each focusing on targets involved in cancers and serious infections. Using these cells, the power of the immune system can be employed to attack cancer cells and cells infected with certain viruses. T-cells may be effective even after failure of multiple other agents, and may avoid the toxicities of current treatments in patients with cancers and serious viral infections. CMV-CTLs and EBV-CTLs are currently in Phase 2 clinical trials and WT1-CTLs are currently in Phase 1 clinical studies. The EBV-, CMV- and WT1-targeted T-cell product candidates share a common technology in which third-party donor-derived white blood cells are collected via leukapheresis (white blood cell collection) and are then enriched for T-cells. The T-cells are exposed to certain antigens (proteins that are recognized and attacked by the immune system), and the resulting activated T-cells are characterized and stored for future therapeutic use in a partially human leukocyte antigen, or HLA, matched patient. MSK has developed banks of these off-the-shelf, target-specific T-cell product candidates suitable for investigational use in patients with a wide range of HLA types.
Via Krishan Maggon
|
At the annual meeting of the American Association of Cancer Researchers, presenters discuss strategies to improve the safety and effectiveness of reengineered T cells in eradicating tumors.
Via Krishan Maggon
The promising clinical results obtained with engineered T cells, including chimeric antigen receptor (CAR) therapy, call for further advancements to facilitate and broaden their applicability. One potentially beneficial innovation is to exploit new T cell sources that reduce the need for autologous cell manufacturing and enable cell transfer across histocompatibility barriers. Here we review emerging T cell engineering approaches that utilize alternative T cell sources, which include virus-specific or T cell receptor-less allogeneic T cells, expanded lymphoid progenitors, and induced pluripotent stem cell (iPSC)-derived T lymphocytes. The latter offer the prospect for true off-the-shelf, genetically enhanced, histocompatible cell therapy products.
Via Krishan Maggon
Abstract The recent successes of adoptive T-cell immunotherapy for the treatment of hematologic malignancies have highlighted the need for manufacturing processes that are robust and scalable for product commercialization. Here we review some of the more outstanding issues surrounding commercial scale manufacturing of personalized-adoptive T-cell medicinal products. These include closed system operations, improving process robustness and simplifying work flows, reducing labor intensity by implementing process automation, scalability and cost, as well as appropriate testing and tracking of products, all while maintaining strict adherence to Current Good Manufacturing Practices and regulatory guidelines. A decentralized manufacturing model is proposed, where in the future patients’ cells could be processed at the point-of-care in the hospital.
Via Krishan Maggon
James P. Allison, who saw the devastating effects of cancer on his family, discovered a way to disable one of its main defenses. It was breakthrough of the year in Science two years ago
Via Krishan Maggon
#TIL's Genetically #Engineered w/Inducible Gene Encoding IL-12 for #Immunotherapy of Metastatic #Melanoma http://t.co/ABuiy4QodT #health Abstract Purpose: Infusion of interleukin-12 (IL-12) can mediate anti-tumor immunity in animal models, yet its systemic administration to patients with cancer results in minimal efficacy and severe toxicity. Here, we evaluated the anti-tumor activity of adoptively transferred human tumor infiltrating lymphocytes (TIL) genetically engineered to secrete single-chain IL-12 selectively at the tumor site. Experimental Design:Thirty-three patients with metastatic melanoma were treated in a cell-dose escalation trial of autologous TIL transduced with a gene encoding a single chain IL-12 driven by a nuclear factor of activated T cells promoter (NFAT.IL12). No IL-2 was administered. Results:The administration of 0.001-0.1 X 109 NFAT.IL12 transduced TIL to 17 patients resulted in a single objective response (5.9%). However, at doses between 0.3-3 X 109 cells, 10 of 16 patients (63%) exhibited objective clinical responses. The responses tended to be short and the administered IL-12 producing cells rarely persisted at one month. Increasing cell doses were associated with high serum levels of IL-12 and gamma-interferon as well as clinical toxicities including liver dysfunction, high fevers and sporadic life threatening hemodynamic instability. Conclusions:In this first-in-man trial, administration of TIL transduced with an inducible IL-12 gene mediated tumor responses in the absence of IL-2 administration using cell doses 10-100 fold lower than conventional TIL. However, due to toxicities, likely attributable to the secreted IL-12, further refinement will be necessary before this approach can be safely utilized in the treatment of cancer patients.
Via Krishan Maggon
#endcancer
#Cancer #Immunotherapy and Breaking Immune Tolerance
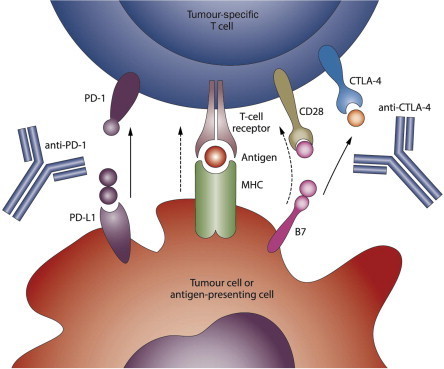
http://t.co/Lt4cqntj4b Abstract Cancer immunotherapy has proven to be challenging as it depends on overcoming multiple mechanisms that mediate immune tolerance to self-antigens. A growing understanding of immune tolerance has been the foundation for new approaches to cancer immunotherapy. Adoptive transfer of immune effectors such as antitumor mAb and chimeric antigen receptor T cells bypasses many of the mechanisms involved in immune tolerance by allowing for expansion of tumor-specific effectors ex vivo. Vaccination with whole tumor cells, protein, peptide, or dendritic cells has proven challenging, yet may be more useful when combined with other cancer immunotherapeutic strategies. Immunomodulatory approaches to cancer immunotherapy include treatment with agents that enhance and maintain T-cell activation. Recent advances in the use of checkpoint blockade to block negative signals and to maintain the antitumor response are particularly exciting. With our growing knowledge of immune tolerance and ways to overcome it, combination treatments are being developed, tested, and have particular promise. One example is in situimmunization that is designed to break tolerance within the tumor microenvironment. Progress in all these areas is continuing based on clear evidence that cancer immunotherapy designed to overcome immune tolerance can be useful for a growing number of patients with cancer. Cancer Res; 75(1); 5–10. ©2014 AACR.
Via Krishan Maggon
|



 Your new post is loading...
Your new post is loading...








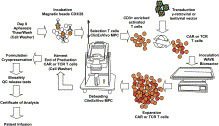







Formula’s R&D plan consists of C.I.K.-CAR.CD19 for B cell malignancies, our lead program, followed closely by the development of C.I.K.-CAR.CD33 for myeloid leukemias. Additionally, Formula plans to develop a C.I.K.-CAR.CD23 program targeting mature B cell malignancies, such as Chronic Lymphocytic Leukemia (CLL), as well as other proprietary targets for solid tumors, pending collaborations and/or adequate financing.
Pre-clinical and clinical research of cancer patients conducted by different research groups suggests that non-targeted C.I.K. cells (not converted into targeted C.I.K. CAR therapy) have tumor killing effects. C.I.K. cell populations also have important memory capability, which supports persistence and continued proliferation of the immune effector cells in-vivo.
Widening Accessibility in Markets
Formula’s C.I.K. CAR platform presently derives PBMCs from healthy donor (i.e. allogeneic) derived blood. Instead of requiring apheresis, Formula’s approach would use a small sample (50 mL) of peripheral blood from a haploidentical or otherwise HLA-matched donor to derive 1X109 C.I.K. cells, which is sufficient for the purpose of C.I.K. CAR therapy. Because physicians cannot predict which patient would render enough or healthy enough PBMCs for CAR-T therapy, Formula believes that having a guaranteed starting dose through donor-derived CAR immunotherapy, without causing clinically unacceptable GvHD, provides the patient and physician with a superior alternative to autologous CAR-T.
NON-VIRAL genetic modification of C.I.K. cell population
Historically, CAR-T cells have been genetically engineered using lenti-viral or retro-viral transfection methods. These methods have been used successfully in specialized laboratory settings for small-scale production. Distinct from most commercially developed CAR therapies by others, Formula adopts a proprietary non-viral transfection method for the creation of CAR-based therapies (C.I.K. CAR) and T cell Receptor (TCR)-based therapies (C.I.K. TCR). Non-viral methods of stable gene transfer have recently been developed, as alternatives to viral vectors, with the purpose to overcome practical, regulatory and manufacturing scale-up limitations related to viral transfection methods. Also, the expression cassette of the non-viral transfection method is integrated by a non-homologous recombinant mechanism, resulting in a safer, random gene insertion, compared to retro-viral vectors that display a marked tendency to target gene promoters, the latter of which leads to an increased probability to deregulate the expression of the targeted genes. The proprietary manufacturing of Formula’s C.I.K. CAR may offer a more streamlined and less complicated route to commercialization.