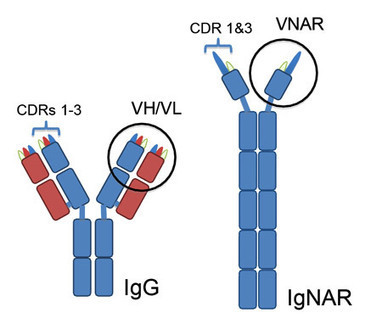
Sharks are the most evolutionarily ancient animal species to possess an adaptive immune system similar to humans, including the production of antibodies against invading pathogens. These ancestral antibodies called IgNARs for Immunoglobulin New Antigen Receptors have a number of remarkable and unique properties. IgNARs evolved as heavy chain only antibodies without the associated light chains found in human antibodies. Their individual variable domains (known as VNARs) are highly soluble compared to the human variable domains, which on their own are generally insoluble and aggregate. Additionally, VNARs are particularly stable and bind antigens under conditions that match, which would disrupt the integrity of most mammalian antibodies. Furthermore, shark antibodies are believed to be particularly stable since they evolved under the high osmolarity of shark blood, which is maintained by the protein denaturant urea.
Only a very small region at the tip of an antibody directly binds to an antigen and sharks have evolved a unique way of engaging target antigens. The diversity of human antibodies is generated by a complex of two variable domains (VH and VL) each of which has three sites of contact called complementarity-determining regions (CDR 1-3). Thus, antibodies rely on contributions from up to 6 CDRs, which create a relatively flat surface of for antigen binding. By contrast, VNAR single domains of shark antibodies lack a CDR2 and concentrate diversity in an extended CDR3 loop supported by a smaller CDR1 that preferentially seeks out cavities and buried epitopes. Additionally, the position of disulfide bridges that affect the stability, flexibility and orientation of the CDR3 loop create diverse isoforms (called type I, II and IV) that can recognize a wide array of antigens.
VNARs are the smallest known immunoglobulin-based antigen binding domains that can have agonistic or antagonistic effects on their own. Their unique combination of structural and biophysical properties makes them attractive building blocks for drug discovery applications, particularly in situations where monoclonal antibodies have proven to be less than ideal. Such examples include perturbation of protein-protein interactions and for targeting difficult but important target classes such as transporters, ion channels and G protein-coupled receptors and cell-surface carbohydrates. A new generation of modular therapeutic agents can be custom built with innovative functions beyond the reach of classical antibodies for the treatment of a broad range of human diseases.
Via Krishan Maggon



 Your new post is loading...
Your new post is loading...