WHITEHOUSE STATION, N.J.--(BUSINESS WIRE)--Merck (NYSE:MRK), known as MSD outside the United States and Canada, today announced that the U.S.
Food and Drug Administration (FDA) has approved KEYTRUDA® (pembrolizumab) at a dose of 2 mg/kg every three weeks for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. This indication is approved under accelerated approval based on tumor response rate and durability of response. An improvement in survival or disease-related symptoms has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
KEYTRUDA is the first anti-PD-1 (programmed death receptor-1) therapy approved in the United States and received FDA’s Breakthrough Therapy designation for advanced melanoma, which was granted based on the significance of early study findings and the unmet medical need. For the recommended 2 mg/kg dose based on data in 89 patients, the overall response rate was 24 percent (95% CI: 15, 34), with one complete response and 20 partial responses (21/89). At the time of analysis, 86 percent (18/21) of patients with objective responses had ongoing responses with durations ranging from 1.4+ to 8.5+ months, including eight patients with ongoing responses of 6 months or longer. Fourteen percent (3/21) had progression of disease 2.8, 2.9, and 8.2 months after initial response.
KEYTRUDA is a humanized monoclonal antibody that works by increasing the ability of the body’s immune system to fight advanced melanoma. KEYTRUDA blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, and may affect both tumor cells and healthy cells. Immune-mediated adverse reactions occurred with KEYTRUDA including pneumonitis, colitis, hepatitis, hypophysitis, nephritis, hyperthyroidism, and hypothyroidism. Based on the severity of the adverse reaction, KEYTRUDA should be withheld or discontinued and corticosteroids administered. Based on its mechanism of action, KEYTRUDA may cause fetal harm when administered to a pregnant woman. Female patients of reproductive potential should be advised of the potential hazard to a fetus. For more information regarding immune-mediated adverse reactions and use in pregnancy, see “Selected Important Safety Information” below.
Merck is conducting ongoing Phase 2 and 3 clinical studies in advanced melanoma, which are designed to provide further confirmatory evidence for KEYTRUDA in this indication. Merck plans to make KEYTRUDA available within one week from today’s FDA approval.
Study Cohort Supporting the Accelerated FDA Approval of Single-Agent KEYTRUDA
The approval of KEYTRUDA was based on data from a multi-center, open-label, randomized, dose-comparative study cohort of the ongoing KEYNOTE-001 Phase 1b trial in patients with unresectable or metastatic melanoma and progression of disease. Key eligibility criteria included prior treatment with ipilimumab (two or more doses at 3 mg/kg or higher) and a BRAF or MEK inhibitor, if BRAF V600 mutation positive; and disease progression within 24 weeks following the last dose of ipilimumab. Patients were randomized to receive 2 mg/kg (n=89) or 10 mg/kg (n=84) of KEYTRUDA every 3 weeks until unacceptable toxicity or disease progression. The major efficacy outcome measures were confirmed overall response rate as assessed by blinded independent central review using Response Evaluation Criteria in Solid Tumors (RECIST 1.1) and duration of response. Tumor response was assessed every 12 weeks.
Selected Important Safety Information for KEYTRUDA
Pneumonitis occurred in 12 (2.9%) of 411 patients, including Grade 2 or 3 cases in 8 (1.9%) and 1 (0.2%) patients respectively, receiving KEYTRUDA. Monitor patients for signs and symptoms of pneumonitis. Evaluate suspected pneumonitis with radiographic imaging. Administer corticosteroids for Grade 2 or greater pneumonitis. Withhold KEYTRUDA for Grade 2; permanently discontinue KEYTRUDA for Grade 3 or 4 pneumonitis.
Colitis (including microscopic colitis) occurred in 4 (1%) of 411 patients, including Grade 2 or 3 cases in 1 (0.2%) and 2 (0.5%) patients respectively, receiving KEYTRUDA. Monitor patients for signs and symptoms of colitis. Administer corticosteroids for Grade 2 or greater colitis. Withhold KEYTRUDA for Grade 2 or 3; permanently discontinue KEYTRUDA for Grade 4 colitis.
Hepatitis (including autoimmune hepatitis) occurred in 2 (0.5%) of 411 patients, including a Grade 4 case in 1 (0.2%) patient, receiving KEYTRUDA. Monitor patients for changes in liver function. Administer corticosteroids for Grade 2 or greater hepatitis and, based on severity of liver enzyme elevations, withhold or discontinue KEYTRUDA.
Hypophysitis occurred in 2 (0.5%) of 411 patients, including a Grade 2 case in 1 and a Grade 4 case in 1 (0.2% each) patient, receiving KEYTRUDA. Monitor for signs and symptoms of hypophysitis. Administer corticosteroids for Grade 2 or greater hypophysitis. Withhold KEYTRUDA for Grade 2; withhold or discontinue for Grade 3; and permanently discontinue KEYTRUDA for Grade 4 hypophysitis.
Nephritis occurred in 3 (0.7%) patients receiving KEYTRUDA, consisting of one case of Grade 2 autoimmune nephritis (0.2%) and two cases of interstitial nephritis with renal failure (0.5%), one Grade 3 and one Grade 4. Monitor patients for changes in renal function. Administer corticosteroids for Grade 2 or greater nephritis. Withhold KEYTRUDA for Grade 2; permanently discontinue KEYTRUDA for Grade 3 or 4 nephritis.
Hyperthyroidism occurred in 5 (1.2%) of 411 patients, including Grade 2 or 3 cases in 2 (0.5%) and 1 (0.2%) patients respectively, receiving KEYTRUDA. Hypothyroidism occurred in 34 (8.3%) of 411 patients, including a Grade 3 case in 1 (0.2%) patient, receiving KEYTRUDA. Thyroid disorders can occur at any time during treatment. Monitor patients for changes in thyroid function (at the start of treatment, periodically during treatment, and as indicated based on clinical evaluation) and for clinical signs and symptoms of thyroid disorders. Administer corticosteroids for Grade 3 or greater hyperthyroidism. Withhold KEYTRUDA for Grade 3; permanently discontinue KEYTRUDA for Grade 4 hyperthyroidism. Isolated hypothyroidism may be managed with replacement therapy without treatment interruption and without corticosteroids.
The following clinically significant, immune-mediated adverse reactions occurred in less than 1% of patients treated with KEYTRUDA: exfoliative dermatitis, uveitis, arthritis, myositis, pancreatitis, hemolytic anemia, partial seizures arising in a patient with inflammatory foci in brain parenchyma, adrenal insufficiency, myasthenic syndrome, optic neuritis, and rhabdomyolysis.
Based on its mechanism of action, KEYTRUDA may cause fetal harm when administered to a pregnant woman. If used during pregnancy, or if the patient becomes pregnant during treatment, apprise the patient of potential hazard to a fetus. Advise females of reproductive potential to use highly effective contraception during treatment and for 4 months after the last dose of KEYTRUDA.
KEYTRUDA was discontinued for adverse reactions in 6% of 89 patients who received the recommended dose of 2 mg/kg and 9% of 411 patients across all doses studied. Serious adverse reactions occurred in 36% of patients receiving KEYTRUDA. The most frequent serious adverse drug reactions reported in 2% or more of patients were renal failure, dyspnea, pneumonia, and cellulitis.
The most common adverse reactions (reported in ≥20% of patients) were fatigue (47%), cough (30%), nausea (30%), pruritus (30%), rash (29%), decreased appetite (26%), constipation (21%), arthralgia (20%), and diarrhea (20%).
The recommended dose of KEYTRUDA is 2 mg/kg administered as an intravenous infusion over 30 minutes every three weeks until disease progression or unacceptable toxicity. No formal pharmacokinetic drug interaction studies have been conducted with KEYTRUDA.
It is not known whether KEYTRUDA is excreted in human milk. Because many drugs are excreted in human milk, instruct women to discontinue nursing during treatment with KEYTRUDA. Safety and effectiveness of KEYTRUDA have not been established in pediatric patients.
Commitment to Access for KEYTRUDA
Merck is committed to making KEYTRUDA accessible to patients. Reimbursement support for eligible patients receiving KEYTRUDA, including help with out-of-pocket costs and co-pay assistance, is available through The Merck Access Program. For eligible patients who are uninsured, financial assistance is available through Merck’s patient assistance program. More information is available by calling 1-855-257-3932 or visiting www.merckaccessprogram-keytruda.com.
About KEYTRUDA
KEYTRUDA (pembrolizumab) is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2. By binding to the PD-1 receptor and blocking the interaction with the receptor ligands, KEYTRUDA releases the PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response.
Via
Krishan Maggon



 Your new post is loading...
Your new post is loading...


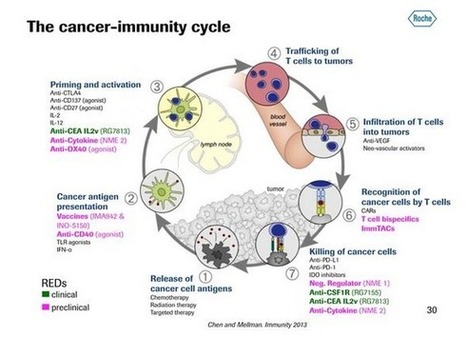


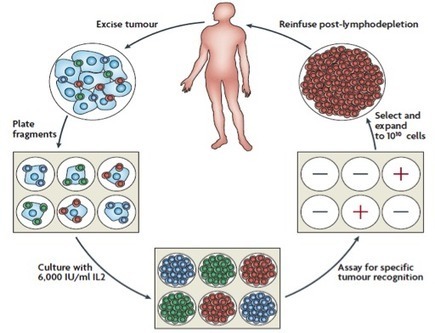


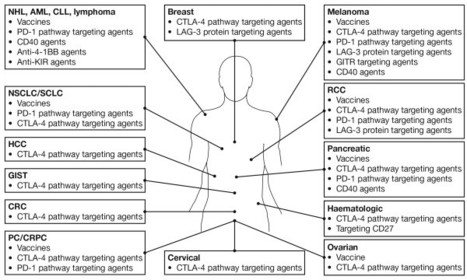







PNAS
Emanuela Romano, doi: 10.1073/pnas.1417320112
Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patientsEmanuela Romanoa,b,c,1, Monika Kusio-Kobialkab, Periklis G. Foukasc,d, Petra Baumgaertnerc,Christiane Meyerc, Pierluigi Ballabenie, Olivier Michielina,c, Benjamin Weidef, Pedro Romeroc, andDaniel E. SpeisercAuthor Affiliations
Edited by Ira Mellman, Genentech, Inc., South San Francisco, CA, and approved March 30, 2015 (received for review September 9, 2014)
PNAS
Emanuela Romano, doi: 10.1073/pnas.1417320112
Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patientsEmanuela Romanoa,b,c,1, Monika Kusio-Kobialkab, Periklis G. Foukasc,d, Petra Baumgaertnerc,Christiane Meyerc, Pierluigi Ballabenie, Olivier Michielina,c, Benjamin Weidef, Pedro Romeroc, andDaniel E. Speiserc
Author Affiliations
Edited by Ira Mellman, Genentech, Inc., South San Francisco, CA, and approved March 30, 2015 (received for review September 9, 2014)