 Your new post is loading...
 Your new post is loading...
SHANGHAI, China and PALO ALTO, Calif., June 9, 2015 /GlobeNewswire/ — Cellular Biomedicine Group Inc. (NASDAQ: CBMG) (“CBMG” or the “Company”), a biomedicine firm engaged in the development of new treatments for degenerative and cancerous diseases, today announced that it has entered into a definitive agreement to acquire from Blackbird Bio Finance (“BB”) University of South Florida’s (“Licensor”) next generation GVAX vaccine’s (“CD40LGVAX”) related technologies and know-how for an initial consideration of $2.5 million in cash and $1.75 million in shares of the Company’s Common Stock. The per share price will be based on the 20-day volume weighted average price (“VWAP”) of the Company’s Common Stock upon the closing of the acquisition. CBMG will pay potentially more than $25 million in future milestones and royalty payments. As part of the transaction, CBMG will be the exclusive global licensee of the Licensor’s related technologies and know-how, the progeny manufacturing rights with access to a master vaccine bank originating from the University of South Florida (“USF”). The inventor of CD40LGVAX, Scott Antonia, MD, Ph.D. is currently the Department Chair of Thoracic Oncology and Program Leader of the Immuno-oncology Program at the Moffitt Cancer Center. Dr. Antonia ranks among the foremost experts in the world of immuno-oncology and is an active collaborator with large pharmaceutical companies. He is recognized as one of the world’s leading authorities in the treatment of lung cancer with immunotherapeutics and has recently joined the Company’s Scientific Advisory Board. Given the positive Phase I results of CD40LGVAX alone in non-small cell lung cancer (NSCLC), Dr. Antonia plans to combine the CD40LGVAX with a checkpoint inhibitor, anti-PD1 monoclonal antibody, Nivolumab, in a three patient lead-in Phase I clinical trial followed by a randomized Phase II clinical trial in the U.S. to evaluate the safety and efficacy of the combination in patients with Stage 4 unresectable non-small cell lung cancer. The clinical trials are expected to commence in the second half of 2015.
Via Krishan Maggon
Findings The anti-PD-1 and anti-PD-L1 agents have been reported to have impressive antitumor effects in several malignancies, including melanoma. The greatest clinical activity in unselected patients has been seen in melanoma. Tumor expression of PD-L1 is a suggestive, but inadequate, biomarker predictive of response to immune-checkpoint blockade. However, tumors expressing little or no PD-L1 are less likely to respond to PD-1 pathway blockade. Combination checkpoint blockade with PD-1 plus cytotoxic T-lymphocyte antigen (CTLA)-4 blockade appears to improve response rates in patients who are less likely to respond to single-checkpoint blockade. Toxicity with PD-1 blocking agents is less than the toxicity with previous immunotherapies (eg, interleukin 2, CTLA-4 blockade). Certain adverse events can be severe and potentially life threatening, but most can be prevented or reversed with close monitoring and appropriate management. Implications This family of immune-checkpoint inhibitors benefits not only patients with metastatic melanoma but also those with historically less responsive tumor types. Although a subset of patients responds to single-agent blockade, the initial trial of checkpoint-inhibitor combinations has reported a potential to improve response rates. Combination therapies appear to be a means of increasing response rates, albeit with increased immune-related adverse events. As these treatments become available to patients, education regarding the recognition and management of immune-related effects of immune-checkpoint blockade will be essential for maximizing clinical benefit.
Via Krishan Maggon
This review discusses the development of targeted and immune therapies for advanced melanoma, reviews current patient management, and highlights future directions.
Via Krishan Maggon
(2015). Macrophages are critical effectors of antibody therapies for cancer. mAbs: Vol. 7, No. 2, pp. 303-310.
Via Krishan Maggon
Abstract Interleukin (IL)-15 is one of the most promising molecules to be used in antitumor immune therapy, as it is able to stimulate the main killer cells of both the innate and adaptive immune system. Although this cytokine can be used as a stand-alone immunotherapeutic agent, IL-15 will probably be most efficient in combination with other strategies to overcome high tumor burden, immune suppression of the tumor microenvironment and/or the short half-life of IL-15. In this review, we will discuss the combination strategies with IL-15 that have been tested to date in different animal tumor models, which include chemotherapy, other immunostimulatory cytokines, targeted therapy, adoptive cell transfer and gene therapy. In addition, we give an overview of IL-15 combination therapies that are currently tested in clinical studies to treat patients with hematological or advanced solid tumors.
Via Krishan Maggon
James P. Allison, who saw the devastating effects of cancer on his family, discovered a way to disable one of its main defenses. It was breakthrough of the year in Science two years ago
Via Krishan Maggon
TUSTIN, CA -- (Marketwired) -- 02/09/15 -- Peregrine Pharmaceuticals, Inc. (NASDAQ: PPHM) (NASDAQ: PPHMP) today announced preclinical data presentations showing that the PS-targeting antibody equivalent to bavituximab combined with an anti-PD-1 antibody displayed statistically significant improvement in tumor fighting immune cells, activation signals and cytokines in a model of melanoma compared to anti-PD-1 alone. Moreover, cells that suppress the immune system from recognizing tumors, such as myeloid-derived suppressor cells (MDSCs), were reduced by more than 40% in the combination with the PS-targeting antibody versus anti-PD-1 alone. These data, further validating the immune-stimulatory mechanism of bavituximab, are outlined in an oral and poster presentation byBruce Freimark, Ph.D., director, preclinical oncology research at Peregrine, to be made at the Keystone Tumor Immunology: Multidisciplinary Science Driving Combination Therapy meeting being held February 8-13, 2015 in Banff, Alberta, Canada. Peregrine's lead PS-targeting antibody, bavituximab, is currently being evaluated in second-line non-small cell lung cancer (NSCLC) as part of the SUNRISE pivotal Phase III clinical trial.
In the presentations titled: "Antibody-Mediated Blockade of Phosphatidylserine Enhances the Anti-Tumor Activity of Immune Checkpoint Inhibitors by Affecting Myeloid-Derived Suppressor Cells (MDSC) and Lymphocyte Populations in the Tumor Microenvironment", Dr. Freimark and his research group, along with colleagues from the University of Texas Southwestern Medical Center led by Xianming Huang, Ph.D., demonstrate that in immunocompetent preclinical models of breast cancer and melanoma, the combination of PS-targeting antibodies and anti-CTLA-4 and anti-PD1 antibodies demonstrate statistically significant anti-tumor responses than either anti-CTLA-4 or anti-PD-1 antibody alone. New data presented show statistically significant changes in levels of tumor infiltrating lymphocytes (TILs), a type of white blood cell implicated in killing tumor cells, in the PS-targeting and anti-PD-1 combination group over single treatment alone in a melanoma model. Specifically, data show increases in a number of markers used to determine immune activation, including CD3 and CD8 cells expressing PD-1, Lag-3 and CD137 (4-1BB). Furthermore, data show that CD8 T cells in the tumor had increased production of IFN-gamma and TNF-α, both known to assist in promoting immune activation and Granzyme-B which is involved in direct tumor killing.
Via Krishan Maggon
The better understanding of the mechanism in which
the immune system responds to the developing cancer provided the outcome in a
new era in cancer immunotherapy.
Via Krishan Maggon
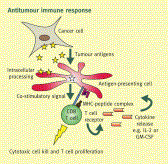
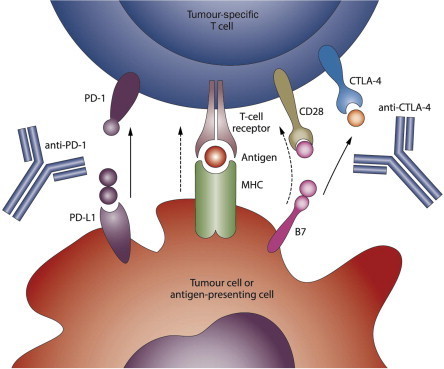
Dysfunctional T cells can render the immune system unable to eliminate infections and cancer. Therapeutic targeting of the surface receptors that inhibit T cell function has begun to show remarkable success in clinical trials. In this Review, we discuss the potential mechanisms of action of the clinical agents that target two of these receptors, programmed cell death protein 1 (PD1) and lymphocyte activation gene 3 protein (LAG3). We also suggest correlative studies that may define the predominant mechanisms of action and identify predictive biomarkers.
Via Krishan Maggon
|
There are many reasons to be excited by the science being presented at this year’s American Society for Clinical Oncology meeting. The data...
Via Krishan Maggon
(Reuters) - Worldwide spending on cancer medicines reached $100 billion in 2014, an increase of 10.3 percent from 2013 and up from $75 billion five years earlier, according to IMS Health's Global Oncology...
Via Krishan Maggon
Monoclonal antibodies (mAbs) have inaugurated the concepts of tumor-targeted therapy and personalized medicine. A new family of mAbs is currently emerging in the clinic, which target immune cells rather than cancer cells. These immune-targeted therapies have recently demonstrated long-term tumor responses in adults with refractory/relapsing metastatic solid tumors. Pediatric cancers are different from their adult counterparts in terms of histological features and immune infiltrates. However, the same immune checkpoint targets can be expressed within the microenvironment of pediatric tumors. The benefits of immune checkpoint blockade in pediatric cancers are currently under evaluation in early phase clinical trials. Pediatr Blood Cancer © 2015 Wiley Periodicals, Inc.
Via Krishan Maggon
Abstract Immunotherapy for the treatment of cancer is rapidly evolving from therapies that globally and non-specifically simulate the immune system to more targeted activation of individual components of the immune system. The net result of this targeted approach is decreased toxicity and increased efficacy of immunotherapy. More specifically, therapies that inhibit the interaction between programmed death ligand 1 (PD-L1), present on the surface of tumor or antigen-presenting cells, and programmed death 1 (PD-1), present on the surface of activated lymphocytes, are generating much excitement and enthusiasm, even in malignancies that are not traditionally considered to be immunogenic. Herein, we review the current landscape of anti-PD-1 and anti-PD-L1 therapies in the world of oncology. We have performed a comprehensive literature search on the data available through PubMed, Medline, Scopus, the ClinicalTrials.gov registry, and abstracts from major oncology meetings in order to summarize the clinical data of anti-PD-1/PD-L1 therapies.
Via Krishan Maggon
Abstract In recent years, immune checkpoints that maintain physiologic self-tolerance have been implicated in the down-regulation of anti-tumor immunity. Efforts to restore latent anti-tumor immunity have focused on antibody-based interventions targeting CTL antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on T lymphocytes and its principal ligand (PD-L1) on tumor cells. Ipilimumab, an antibody targeting CTLA-4, appears to restore tumor immunity at the priming phase, whereas anti-PD-1/PD-L1 antibodies restore immune function in the tumor microenvironment. Although ipilimumab can produce durable long-term responses in patients with advanced melanoma, it is associated with significant immune-related toxicities. By contrast, antibodies targeting either PD-1 or PD-L1 have produced significant anti-tumor activity with considerably less toxicity. Activity was seen in patients with melanoma and renal cancer, as well as those with non-small-cell lung, bladder and head and neck cancers, tumors not previously felt to be sensitive to immunotherapy. The tolerability of PD-1-pathway blockers and their unique mechanism of action have made them ideal backbones for combination regimen development. Combination approaches involving cytotoxic chemotherapy, anti-angiogenic agents, alternative immune-checkpoint inhibitors, immunostimulatory cytokines and cancer vaccines are currently under clinical investigation. Current efforts focus on registration trials of single agents and combinations in various diseases and disease settings and identifying predictive biomarkers of response.
Via Krishan Maggon
Abstract The concept of immunotherapy as a modality to treat cancer was recognized more than a hundred years ago. High-dose interleukin-2 (IL-2) was one of the first agents to demonstrate that the host's immune system can be harnessed to treat even advanced malignancy, as was shown in a subset of patients with renal cancer and melanoma. Many tumours are immunogenic and provoke a host immune response, but this is normally not sufficient to overcome host tolerance. For decades now, researchers have tried various methods to enhance host immunological responses, such as the use of non-specific immunotherapeutic cytokines, tumour vaccines, adoptive immunotherapy and the use of monoclonal antibodies against a wide variety of molecules. This review discusses the principles of the various types of immune therapy and focuses on some of the recent developments and successes in treatment. The article concentrates on the applications of immunotherapy in solid tumours, though it has immense value in haematological cancers.
Via Krishan Maggon
#endcancer
#Cancer #Immunotherapy and Breaking Immune Tolerance
http://t.co/Lt4cqntj4b Abstract Cancer immunotherapy has proven to be challenging as it depends on overcoming multiple mechanisms that mediate immune tolerance to self-antigens. A growing understanding of immune tolerance has been the foundation for new approaches to cancer immunotherapy. Adoptive transfer of immune effectors such as antitumor mAb and chimeric antigen receptor T cells bypasses many of the mechanisms involved in immune tolerance by allowing for expansion of tumor-specific effectors ex vivo. Vaccination with whole tumor cells, protein, peptide, or dendritic cells has proven challenging, yet may be more useful when combined with other cancer immunotherapeutic strategies. Immunomodulatory approaches to cancer immunotherapy include treatment with agents that enhance and maintain T-cell activation. Recent advances in the use of checkpoint blockade to block negative signals and to maintain the antitumor response are particularly exciting. With our growing knowledge of immune tolerance and ways to overcome it, combination treatments are being developed, tested, and have particular promise. One example is in situimmunization that is designed to break tolerance within the tumor microenvironment. Progress in all these areas is continuing based on clear evidence that cancer immunotherapy designed to overcome immune tolerance can be useful for a growing number of patients with cancer. Cancer Res; 75(1); 5–10. ©2014 AACR.
Via Krishan Maggon
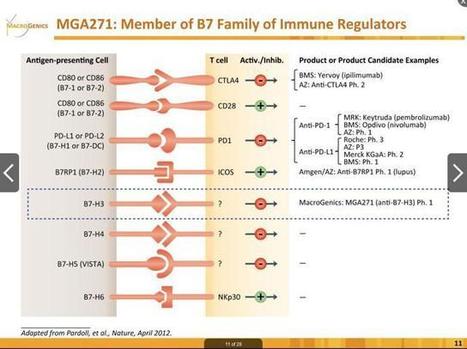
Checkpoint inhibitors are a big deal. What started with ipilimumab has now grown into a class of drugs which are being pursued vigorously by nearly every major pharmaceutical company in the world. In this crowded space, it's worth noting that B7-H3 is only being pursued by one company, MacroGenics (NASDAQ:MGNX).
Via Krishan Maggon
|



 Your new post is loading...
Your new post is loading...









![Clinical blockade of PD1 and LAG3 [mdash] potential mechanisms of action : Nature Reviews Immunology : Nature Publishing Group | Immunology and Biotherapies | Scoop.it](https://img.scoop.it/Z7M6OcSPx7hB-JNuLC7aKjl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)
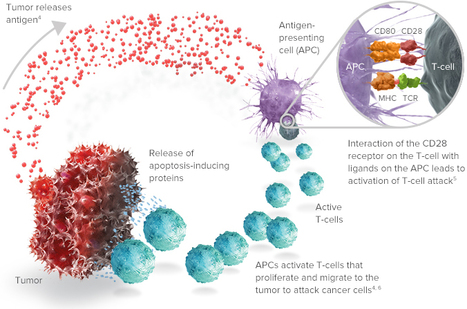








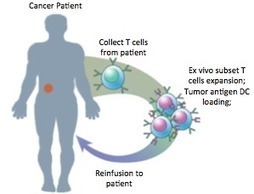
ABOUT CD40LGVAX
CD40LGVAX is a vaccine in which irradiated allogeneic lung adenocarcinoma cells are combined with a bystander cell line transfected with hCD40L and hGM-CSF. The key differentiator is the transfection of the bystander cell line with GM-CSF and CD40L. Both GM-CSF and CD40L can activate dendritic cells (DC). Nivolumab, an anti-PD-1 monoclonal antibody, enhances cytotoxic T cell activity by blocking the interaction between PD-1 and its receptors. The vaccine has previously been tested in a Phase I trial, and has shown encouraging efficacy and toxicity profile. In the United States, the Food and Drug Administration (“FDA”) has approved Nivolumab for treatment of patients with melanoma and advanced squamous NSCLC who have progressed on or after platinum-based chemotherapy. About 25% to 30% of all NSCLC are squamous. By combining CD40LGVAX with an anti-PD1 monoclonal antibody, the approach is expected to further boost the body’s immune system to kill cancer cells. Given the strength of both products and potential synergistic mechanism of action, this potential combination may provide more clinical benefit to NSCLC than either product alone.