 Your new post is loading...
 Your new post is loading...
Abstract Lung cancer remains the most common cause of cancer-related deaths in the United States, with SEER data showing lung cancer accounting for 29% of all male-related cancer mortality and 26% of all female-related mortality. Patients with small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) who have localized disease both have 5-year survival rates of 52.2%, whereas patients with metastatic disease have 5-year survival rates of only 3.7%. Traditional anti-cancer therapies (surgery, radiotherapy, and chemotherapy) have limited effectiveness in curbing progression. However, advances in immunology and molecular biology in the past two decades have resulted in improved prognosis for those with SCLC and NSCLC, although novel therapies are still needed to make significant improvements in median overall and progression-free survival rates. Notable progress on the importance of tumor immunology has included work on immune surveillance, antigenic targets, and immune checkpoints. Immunotherapies, including vaccines, which can induce antitumor responses by harnessing the power of the immune system, may help to fill this void, and the cancer vaccine continues to be studied as adjunctive therapy. Here, we review recently reported results from clinical trials as well as the possible future roles of vaccine therapy in the treatment of SCLC and NSCLC patients.
Via Krishan Maggon
Findings The anti-PD-1 and anti-PD-L1 agents have been reported to have impressive antitumor effects in several malignancies, including melanoma. The greatest clinical activity in unselected patients has been seen in melanoma. Tumor expression of PD-L1 is a suggestive, but inadequate, biomarker predictive of response to immune-checkpoint blockade. However, tumors expressing little or no PD-L1 are less likely to respond to PD-1 pathway blockade. Combination checkpoint blockade with PD-1 plus cytotoxic T-lymphocyte antigen (CTLA)-4 blockade appears to improve response rates in patients who are less likely to respond to single-checkpoint blockade. Toxicity with PD-1 blocking agents is less than the toxicity with previous immunotherapies (eg, interleukin 2, CTLA-4 blockade). Certain adverse events can be severe and potentially life threatening, but most can be prevented or reversed with close monitoring and appropriate management. Implications This family of immune-checkpoint inhibitors benefits not only patients with metastatic melanoma but also those with historically less responsive tumor types. Although a subset of patients responds to single-agent blockade, the initial trial of checkpoint-inhibitor combinations has reported a potential to improve response rates. Combination therapies appear to be a means of increasing response rates, albeit with increased immune-related adverse events. As these treatments become available to patients, education regarding the recognition and management of immune-related effects of immune-checkpoint blockade will be essential for maximizing clinical benefit.
Via Krishan Maggon
Abstract Cancer vaccines are designed to promote tumor specific immune responses, particularly cytotoxic CD8 positive T cells that are specific to tumor antigens. The earliest vaccines, which were developed in 1994-95, tested non-mutated, shared tumor associated antigens that had been shown to be immunogenic and capable of inducing clinical responses in a minority of people with late stage cancer. Technological developments in the past few years have enabled the investigation of vaccines that target mutated antigens that are patient specific. Several platforms for cancer vaccination are being tested, including peptides, proteins, antigen presenting cells, tumor cells, and viral vectors. Standard of care treatments, such as surgery and ablation, chemotherapy, and radiotherapy, can also induce antitumor immunity, thereby having cancer vaccine effects. The monitoring of patients’ immune responses at baseline and after standard of care treatment is shedding light on immune biomarkers. Combination therapies are being tested in clinical trials and are likely to be the best approach to improving patient outcomes.
Via Krishan Maggon
The MaxCyte GT® Flow Transfection System is a universal platform technology for rapid, automated loading of CAR (chimeric antigen receptor) mRNA into peripheral blood mononuclear cells using closed-system aseptic processing.
Via Krishan Maggon
Tumor-specific immunotherapy holds the promise of eradicating malignant tumors with exquisite precision without additional toxicity to standard treatments.
Via Krishan Maggon
Highlights • T cells undergo metabolic remodeling to support their function. • Metabolic pathways impact on T cell differentiation decisions and function in the periphery. • Manipulating metabolic microenvironments may enhance T cell function in cancer. • Metabolic pathways could be targeted for the treatment of human disease. In the past several years a wealth of evidence has emerged illustrating how metabolism supports many aspects of T cell biology, as well as how metabolic changes drive T cell differentiation and fate. We outline developing principles in the regulation of T cell metabolism, and discuss how these processes are affected in settings of inflammation and cancer. In this context we discuss how metabolic pathways might be manipulated for the treatment of human disease, including how metabolism may be targeted to prevent T cell dysfunction in inhospitable microenvironments, to generate more effective adoptive cellular immunotherapies in cancer, and to direct T cell differentiation and function towards non-pathogenic phenotypes in settings of autoimmunity.
Via Krishan Maggon
Abstract Interleukin (IL)-15 is one of the most promising molecules to be used in antitumor immune therapy, as it is able to stimulate the main killer cells of both the innate and adaptive immune system. Although this cytokine can be used as a stand-alone immunotherapeutic agent, IL-15 will probably be most efficient in combination with other strategies to overcome high tumor burden, immune suppression of the tumor microenvironment and/or the short half-life of IL-15. In this review, we will discuss the combination strategies with IL-15 that have been tested to date in different animal tumor models, which include chemotherapy, other immunostimulatory cytokines, targeted therapy, adoptive cell transfer and gene therapy. In addition, we give an overview of IL-15 combination therapies that are currently tested in clinical studies to treat patients with hematological or advanced solid tumors.
Via Krishan Maggon
Abstract In recent years, immune checkpoints that maintain physiologic self-tolerance have been implicated in the down-regulation of anti-tumor immunity. Efforts to restore latent anti-tumor immunity have focused on antibody-based interventions targeting CTL antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on T lymphocytes and its principal ligand (PD-L1) on tumor cells. Ipilimumab, an antibody targeting CTLA-4, appears to restore tumor immunity at the priming phase, whereas anti-PD-1/PD-L1 antibodies restore immune function in the tumor microenvironment. Although ipilimumab can produce durable long-term responses in patients with advanced melanoma, it is associated with significant immune-related toxicities. By contrast, antibodies targeting either PD-1 or PD-L1 have produced significant anti-tumor activity with considerably less toxicity. Activity was seen in patients with melanoma and renal cancer, as well as those with non-small-cell lung, bladder and head and neck cancers, tumors not previously felt to be sensitive to immunotherapy. The tolerability of PD-1-pathway blockers and their unique mechanism of action have made them ideal backbones for combination regimen development. Combination approaches involving cytotoxic chemotherapy, anti-angiogenic agents, alternative immune-checkpoint inhibitors, immunostimulatory cytokines and cancer vaccines are currently under clinical investigation. Current efforts focus on registration trials of single agents and combinations in various diseases and disease settings and identifying predictive biomarkers of response.
Via Krishan Maggon
Cancer Gene Therapy is the essential gene therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene therapy for cancer.
Via Krishan Maggon
Abstract The concept of immunotherapy as a modality to treat cancer was recognized more than a hundred years ago. High-dose interleukin-2 (IL-2) was one of the first agents to demonstrate that the host's immune system can be harnessed to treat even advanced malignancy, as was shown in a subset of patients with renal cancer and melanoma. Many tumours are immunogenic and provoke a host immune response, but this is normally not sufficient to overcome host tolerance. For decades now, researchers have tried various methods to enhance host immunological responses, such as the use of non-specific immunotherapeutic cytokines, tumour vaccines, adoptive immunotherapy and the use of monoclonal antibodies against a wide variety of molecules. This review discusses the principles of the various types of immune therapy and focuses on some of the recent developments and successes in treatment. The article concentrates on the applications of immunotherapy in solid tumours, though it has immense value in haematological cancers.
Via Krishan Maggon
TUSTIN, CA -- (Marketwired) -- 02/09/15 -- Peregrine Pharmaceuticals, Inc. (NASDAQ: PPHM) (NASDAQ: PPHMP) today announced preclinical data presentations showing that the PS-targeting antibody equivalent to bavituximab combined with an anti-PD-1 antibody displayed statistically significant improvement in tumor fighting immune cells, activation signals and cytokines in a model of melanoma compared to anti-PD-1 alone. Moreover, cells that suppress the immune system from recognizing tumors, such as myeloid-derived suppressor cells (MDSCs), were reduced by more than 40% in the combination with the PS-targeting antibody versus anti-PD-1 alone. These data, further validating the immune-stimulatory mechanism of bavituximab, are outlined in an oral and poster presentation byBruce Freimark, Ph.D., director, preclinical oncology research at Peregrine, to be made at the Keystone Tumor Immunology: Multidisciplinary Science Driving Combination Therapy meeting being held February 8-13, 2015 in Banff, Alberta, Canada. Peregrine's lead PS-targeting antibody, bavituximab, is currently being evaluated in second-line non-small cell lung cancer (NSCLC) as part of the SUNRISE pivotal Phase III clinical trial.
In the presentations titled: "Antibody-Mediated Blockade of Phosphatidylserine Enhances the Anti-Tumor Activity of Immune Checkpoint Inhibitors by Affecting Myeloid-Derived Suppressor Cells (MDSC) and Lymphocyte Populations in the Tumor Microenvironment", Dr. Freimark and his research group, along with colleagues from the University of Texas Southwestern Medical Center led by Xianming Huang, Ph.D., demonstrate that in immunocompetent preclinical models of breast cancer and melanoma, the combination of PS-targeting antibodies and anti-CTLA-4 and anti-PD1 antibodies demonstrate statistically significant anti-tumor responses than either anti-CTLA-4 or anti-PD-1 antibody alone. New data presented show statistically significant changes in levels of tumor infiltrating lymphocytes (TILs), a type of white blood cell implicated in killing tumor cells, in the PS-targeting and anti-PD-1 combination group over single treatment alone in a melanoma model. Specifically, data show increases in a number of markers used to determine immune activation, including CD3 and CD8 cells expressing PD-1, Lag-3 and CD137 (4-1BB). Furthermore, data show that CD8 T cells in the tumor had increased production of IFN-gamma and TNF-α, both known to assist in promoting immune activation and Granzyme-B which is involved in direct tumor killing.
Via Krishan Maggon
Highlights • The presence of immune infiltrate in tumors has been correlated with a good prognosis following treatment. • Tumor-infiltrating lymphocytes have been utilized for the treatment of melanoma. • To broaden this therapy for other cancers, T cells have been genetically modified with either a T cell receptor or a chimeric antigen receptor. • Potential hurdles and novel strategies will be discussed for realizing the full potential of adoptive immunotherapy becoming a standard of care treatment for cancer.
Via Krishan Maggon
|
Abstract Scientific insights into the human immune system have recently led to unprecedented breakthroughs in immunotherapy. In the twenty-first century, drugs and cell-based therapies developed to bolster humoral and T cell immunity represent an established and growing component of cancer therapeutics. Although natural killer (NK) cells have long been known to have advantages over T cells in terms of their capacity to induce antigen-independent host immune responses against malignancies, their therapeutic potential in the clinic has been largely unexplored. A growing number of scientific discoveries into pathways that both activate and suppress NK cell function, as well as methods to sensitize tumours to NK cell cytotoxicity, have led to the development of numerous pharmacological and genetic methods to enhance NK cell antitumour immunity. These findings, as well as advances in our ability to expand NK cells ex vivoand manipulate their capacity to home to the tumour, have now provided investigators with a variety of new methods and strategies to harness the full potential of NK cell-based cancer immunotherapy in the clinic.
Via Krishan Maggon
There are many reasons to be excited by the science being presented at this year’s American Society for Clinical Oncology meeting. The data...
Via Krishan Maggon
Highlights • CD154 is a co-stimulatory molecule known for its role in inflammatory and autoimmune diseases. • CD154 and its receptor, CD40 are expressed in many types of tumors. • The CD154/CD40 interaction is capable of inducing the proliferation and rescue from apoptosis of tumor cells. • CD154 activates anti-tumoral immunity, and by engaging CD40 may induce apoptosis of tumor cells. • The role of CD154 in cancer immunotherapy was demonstrated in animal models and clinically assessed in patients.
Via Krishan Maggon
At the annual meeting of the American Association of Cancer Researchers, presenters discuss strategies to improve the safety and effectiveness of reengineered T cells in eradicating tumors.
Via Krishan Maggon
"OS & Long-Term Safety of #Nivolumab in Patients With Previously Treated Advanced #NSCLC" JCO http://t.co/TjxJ8tP1Sr Purpose Programmed death 1 is an immune checkpoint that suppresses antitumor immunity. Nivolumab, a fully human immunoglobulin G4 programmed death 1 immune checkpoint inhibitor antibody, was active and generally well tolerated in patients with advanced solid tumors treated in a phase I trial with expansion cohorts. We report overall survival (OS), response durability, and long-term safety in patients with non–small-cell lung cancer (NSCLC) receiving nivolumab in this trial. Patients and Methods Patients (N = 129) with heavily pretreated advanced NSCLC received nivolumab 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. Tumor burden was assessed by RECIST (version 1.0) after each cycle. Results Median OS across doses was 9.9 months; 1-, 2-, and 3-year OS rates were 42%, 24%, and 18%, respectively, across doses and 56%, 42%, and 27%, respectively, at the 3-mg/kg dose (n = 37) chosen for further clinical development. Among 22 patients (17%) with objective responses, estimated median response duration was 17.0 months. An additional six patients (5%) had unconventional immune-pattern responses. Response rates were similar in squamous and nonsquamous NSCLC. Eighteen responding patients discontinued nivolumab for reasons other than progressive disease; nine (50%) of those had responses lasting > 9 months after their last dose. Grade 3 to 4 treatment-related adverse events occurred in 14% of patients. Three treatment-related deaths (2% of patients) occurred, each associated with pneumonitis. Conclusion Nivolumab monotherapy produced durable responses and encouraging survival rates in patients with heavily pretreated NSCLC. Randomized clinical trials with nivolumab in advanced NSCLC are ongoing.
Via Krishan Maggon
(2015). Macrophages are critical effectors of antibody therapies for cancer. mAbs: Vol. 7, No. 2, pp. 303-310.
Via Krishan Maggon
Abstract Immunotherapy for the treatment of cancer is rapidly evolving from therapies that globally and non-specifically simulate the immune system to more targeted activation of individual components of the immune system. The net result of this targeted approach is decreased toxicity and increased efficacy of immunotherapy. More specifically, therapies that inhibit the interaction between programmed death ligand 1 (PD-L1), present on the surface of tumor or antigen-presenting cells, and programmed death 1 (PD-1), present on the surface of activated lymphocytes, are generating much excitement and enthusiasm, even in malignancies that are not traditionally considered to be immunogenic. Herein, we review the current landscape of anti-PD-1 and anti-PD-L1 therapies in the world of oncology. We have performed a comprehensive literature search on the data available through PubMed, Medline, Scopus, the ClinicalTrials.gov registry, and abstracts from major oncology meetings in order to summarize the clinical data of anti-PD-1/PD-L1 therapies.
Via Krishan Maggon
MT @NatRevClinOncol A shed NKG2D ligand that promotes NK cell activation & #tumor rejection http://t.co/fjxcQRyDGS #immunotherapy #cancer Abstract Immune cells, including natural killer (NK) cells, recognize transformed cells and eliminate them in a process termed immunosurveillance. It is thought that tumor cells evade immunosurveillance by shedding membrane ligands that bind to the NKG2D activating receptor on NK cells and/or T cells, and desensitize these cells. In contrast, we show that in mice, shedding of MULT1, a high affinity NKG2D ligand, causes NK cell activation and tumor rejection. Recombinant soluble MULT1 stimulated tumor rejection in mice. Soluble MULT1 functions, at least in part, by competitively reversing a global desensitization of NK cells imposed by engagement of membrane NKG2D ligands on tumor-associated cells, such as myeloid cells. The results overturn conventional wisdom that soluble ligands are inhibitory, and suggest a new approach for cancer immunotherapy.
Via Krishan Maggon
James P. Allison, who saw the devastating effects of cancer on his family, discovered a way to disable one of its main defenses. It was breakthrough of the year in Science two years ago
Via Krishan Maggon
Abstract Dendritic cell (DC) vaccination and cytokine-induced killer (CIK) cell therapy (DC/CIK) have shown limited success in the treatment of advanced non-small cell lung cancer (NSCLC). To investigate the reason for this limited success, the effects of DC/CIK cell therapy on the immune responses of tumor-bearing patients and patients with resected NSCLC were evaluated. In the total 50 patients studied, the serum concentrations of the Th2 cytokines (IL-4 and IL-10) in tumor-bearing patients were significantly higher than those with resected NSCLC before immunotherapy. The post-therapy Th1 cytokine (IFN-γ) level in patients with resected NSCLC significantly increased from the pre-therapy level. In contrast, significantly enhanced post-therapy Th2 cytokine (IL-4 and IL-10) levels were found in tumor-bearing patients. The intracellular staining assay revealed that DC/CIK cell therapy increased the IFN-γ-producing T lymphocyte (CD8+IFN-γ+) frequency in patients with resected NSCLC, but these lymphocytes were not found in tumor-bearing patients. Furthermore, overproduction of vascular endothelial growth factor (VEGF) in tumor-bearing patients showed a statistically positive correlation with IL-4, suggesting that VEGF might be responsible for the predominance of serum Th2 cytokines. In a word, tumor-bearing patients developed a Th2-dominant status that could not be reversed toward Th1 following immunotherapy. A combined regiment of DC vaccination and CIK cell therapy with other treatments to overcome systemic Th2-dominant immune response might improve the current clinical benefit.
Via Krishan Maggon
Cancer immunotherapy utilizing Vγ9Vδ2 T cells has been developed over the past decade. A large number of clinical trials have been conducted on various types of solid tumors as well as hematological malignancies. Vγ9Vδ2 T cell-based immunotherapy can be classified into two categories based on the methods of activation and expansion of these cells. Although the in vivo expansion of Vγ9Vδ2 T cells by phosphoantigens or nitrogen-containing bisphosphonates (N-bis) has been translated to early-phase clinical trials, in which the safety of the treatment was confirmed, problems such as activation-induced Vγ9Vδ2 T cell anergy and a decrease in the number of peripheral blood Vγ9Vδ2 T cells after infusion of these stimulants have not yet been solved. In addition, it is difficult to ex vivo expand Vγ9Vδ2 T cells from advanced cancer patients with decreased initial numbers of peripheral blood Vγ9Vδ2 T cells. In this article, we review the clinical studies and reports targeting Vγ9Vδ2 T cells and discuss the development and improvement of Vγ9Vδ2 T cell-based cancer immunotherapy.
Via Krishan Maggon
Vaccine-based cancer immunotherapy has generated highly variable clinical results due to differing methods of vaccine preparation and variation in patient populations, among other lesser factors. M...
Via Krishan Maggon
|



 Your new post is loading...
Your new post is loading...










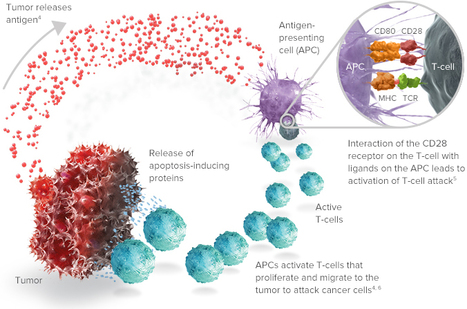
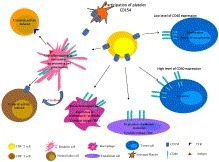









Volume 153, September 2015, Pages 1–9
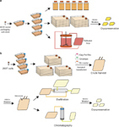
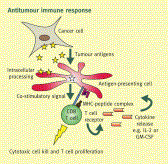
Vaccine immunotherapy in lung cancer: Clinical experience and future directionsMorganna Freeman-Kellera, Jamie Goldmana, Jhanelle Grayb, , doi:10.1016/j.pharmthera.2015.05.004