Chimeric Antigen Receptor (CAR) refers to a novel technology that leverages the immune system in a rapid, potent and direct manner against cancer. CARs are engineered receptors that can target surface molecules of interest expressed on tumor cells. CARs typically engage the target antigen via a single-chain variable fragment (scFv) derived from a monoclonal antibody. Beyond the scFv, the hinge, transmembrane and intracellular signaling domains of CAR make important contributions to the interaction with antigen, assembly of the immunologic synapse, and association of the CAR with other proteins necessary to transduce a robust activation signal.
Formula’s technology is distinctive in that it uses a different kind of immune effector cell for CAR directed therapy, namely Cytokine Induced Killer (C.I.K.) cells (rather than T cells for CAR-T). C.I.K. cells are lymphocytes that also have Natural Killer (NK) cell-like properties, despite the fact that 95% of the C.I.K. cell population has the CD3+, T cell phenotype. The NK-like properties involve immune-cell activation mechanisms different from T cells, in that the NK-like anti-tumor response is non-MHC restricted. Because of these additional features, C.I.K. cells may therefore have a broader therapeutic impact.
Via Krishan Maggon



 Your new post is loading...
Your new post is loading...



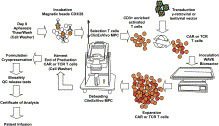







Formula’s R&D plan consists of C.I.K.-CAR.CD19 for B cell malignancies, our lead program, followed closely by the development of C.I.K.-CAR.CD33 for myeloid leukemias. Additionally, Formula plans to develop a C.I.K.-CAR.CD23 program targeting mature B cell malignancies, such as Chronic Lymphocytic Leukemia (CLL), as well as other proprietary targets for solid tumors, pending collaborations and/or adequate financing.
Pre-clinical and clinical research of cancer patients conducted by different research groups suggests that non-targeted C.I.K. cells (not converted into targeted C.I.K. CAR therapy) have tumor killing effects. C.I.K. cell populations also have important memory capability, which supports persistence and continued proliferation of the immune effector cells in-vivo.
Widening Accessibility in Markets
Formula’s C.I.K. CAR platform presently derives PBMCs from healthy donor (i.e. allogeneic) derived blood. Instead of requiring apheresis, Formula’s approach would use a small sample (50 mL) of peripheral blood from a haploidentical or otherwise HLA-matched donor to derive 1X109 C.I.K. cells, which is sufficient for the purpose of C.I.K. CAR therapy. Because physicians cannot predict which patient would render enough or healthy enough PBMCs for CAR-T therapy, Formula believes that having a guaranteed starting dose through donor-derived CAR immunotherapy, without causing clinically unacceptable GvHD, provides the patient and physician with a superior alternative to autologous CAR-T.
NON-VIRAL genetic modification of C.I.K. cell population
Historically, CAR-T cells have been genetically engineered using lenti-viral or retro-viral transfection methods. These methods have been used successfully in specialized laboratory settings for small-scale production. Distinct from most commercially developed CAR therapies by others, Formula adopts a proprietary non-viral transfection method for the creation of CAR-based therapies (C.I.K. CAR) and T cell Receptor (TCR)-based therapies (C.I.K. TCR). Non-viral methods of stable gene transfer have recently been developed, as alternatives to viral vectors, with the purpose to overcome practical, regulatory and manufacturing scale-up limitations related to viral transfection methods. Also, the expression cassette of the non-viral transfection method is integrated by a non-homologous recombinant mechanism, resulting in a safer, random gene insertion, compared to retro-viral vectors that display a marked tendency to target gene promoters, the latter of which leads to an increased probability to deregulate the expression of the targeted genes. The proprietary manufacturing of Formula’s C.I.K. CAR may offer a more streamlined and less complicated route to commercialization.