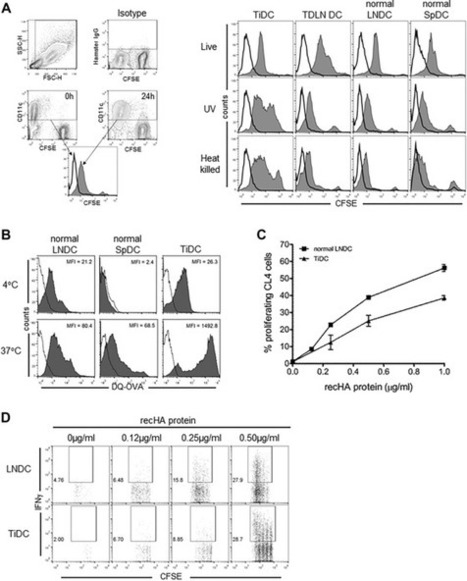
AbstractCross-presentation defines the unique capacity of an APC to present exogenous Ag via MHC class I molecules to CD8+ T cells. DCs are specialized cross-presenting cells and as such have a critical role in antitumor immunity. DCs are routinely found within the tumor microenvironment, but their capacity for endogenous or therapeutically enhanced cross-presentation is not well characterized. In this study, we examined the tumor and lymph node DC cross-presentation of a nominal marker tumor Ag, HA, expressed by the murine mesothelioma tumor AB1-HA. We found that tumors were infiltrated by predominantly CD11b+ DCs with a semimature phenotype that could not cross-present tumor Ag, and therefore, were unable to induce tumor-specific T-cell activation or proliferation. Although tumor-infiltrating DCs were able to take up, process, and cross-present exogenous cell-bound and soluble Ags, this was significantly impaired relative to lymph node DCs. Importantly, however, systemic chemotherapy using gemcitabine reversed the defect in Ag cross-presentation of tumor DCs. These data demonstrate that DC cross-presentation within the tumor microenvironment is defective, but can be reversed by chemotherapy. These results have important implications for anticancer therapy, particularly regarding the use of immunotherapy in conjunction with cytotoxic chemotherapy.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...







Volume 45, Issue 1
AuthorsAlison M. McDonnell, Willem Joost Lesterhuis, Andrea Khong, Anna K. Nowak, Richard A. Lake, Andrew J. Currie, Bruce W. S. Robinson First published: 15 December 2014Full publication historyDOI: 10.1002/eji.201444722View/save citationJanuary 2015
Pages 49–59