Biogen Idec’s PLEGRIDY™(Peginterferon Beta-1a) Approved in the US for the Treatment of Multiple Sclerosis
Reduces Relapses, Disability Progression and Brain Lesions with a Favorable Safety Profile −
− Only Pegylated Interferon in MS, Dosed Once Every Two Weeks –
− Complements Biogen Idec’s Industry-Leading Portfolio of MS Products –
CAMBRIDGE, Mass.Today Biogen Idec (NASDAQ: BIIB) announced that the U.S. Food and Drug Administration (FDA) has approved PLEGRIDYTM (peginterferon beta-1a), a new treatment for people with relapsing forms of multiple sclerosis (RMS). PLEGRIDY, the only pegylated beta interferon approved for use in RMS, is dosed once every two weeks and can be administered subcutaneously with the PLEGRIDY PEN, a new, ready-to-use autoinjector, or a prefilled syringe.
The FDA approval of PLEGRIDY is based on results from one of the largest pivotal studies of beta interferon conducted, ADVANCE, which involved more than 1,500 MS patients. ADVANCE was a two-year, Phase 3, placebo-controlled (in year one) study that evaluated the efficacy and safety of PLEGRIDY administered subcutaneously. The analysis for all primary and secondary efficacy endpoints occurred at the end of year one. After the first year, patients on placebo received PLEGRIDY for the duration of the study.
In the first year of the ADVANCE clinical trial, PLEGRIDY dosed once every two weeks significantly reduced annualized relapse rate (ARR) at one year by 36 percent compared to placebo (p=0.0007). PLEGRIDY reduced the risk of 12-week confirmed disability progression, as measured by the Expanded Disability Status Scale, by 38 percent (p=0.0383) compared to placebo. PLEGRIDY also significantly reduced the number of new gadolinium-enhancing [Gd+] lesions by 86 percent (p<0.0001) and reduced new or newly enlarging T2-hyperintense lesions by 67 percent (p<0.0001) compared to placebo.
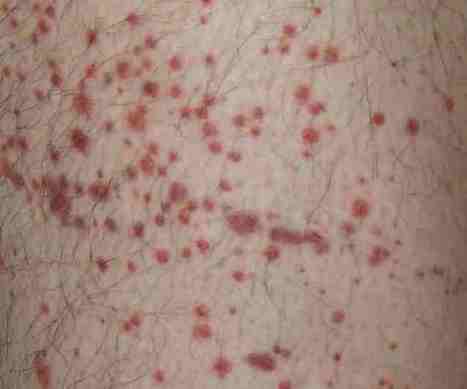
The most common adverse reactions were injection site reaction, flu-like illness, fever, headache, muscle pain, chills, injection site pain, weakness, injection site itching and joint pain. The ADVANCE two-year safety data were consistent with safety results observed in year one.
Via
Krishan Maggon


 Your new post is loading...
Your new post is loading...













Journal of Autoimmunity
Volume 54, November 2014, Pages 1–7
Honoring the contributions of Ruth Arnon and Michael Sela
Review From defining antigens to new therapies in multiple sclerosis: Honoring the contributions of Ruth Arnon and Michael SelaLawrence Steinmana, , Yehuda Shoenfeldb, , , DOI: 10.1016/j.jaut.2014.08.001