Your new post is loading...
 Your new post is loading...
Authors: Ylenia Vittozzi, Thorben Krüger, Adity Majee, Guillaume Née and Stephan Wenkel.
Trends in Plant Science (2024)
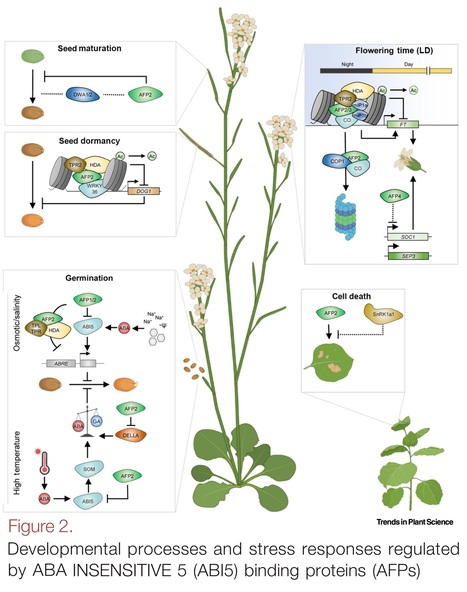
Highlights: The involvement of the ABA INSENSITIVE 5 (ABI5) binding protein family (AFPs), closely related to NOVEL INTERACTOR OF JAZ (NINJA) proteins, has been revealed in several aspects of plant development related to multifaceted plant abscisic acid (ABA) responses. The abundance of AFP proteins is tightly regulated, and they function as a rheostat for ABA responses through several nonmutually exclusive molecular mechanisms. AFP proteins regulate important biological processes in plants, which are tightly controlled by transcriptional repressors and phytohormone-mediated pathways, specifically ABA, gibberellic acid (GA), and jasmonic acid. In today's world, the most pressing concerns are food security and dynamic climatic conditions. Enriching our knowledge regarding plants’ survival and growth strategies through addressing the inherent voids in plant AFP research might foster improvements in various crop species through genetic manipulation.
Abstract: "During the course of terrestrial evolution, plants have developed complex networks that involve the coordination of phytohormone signalling pathways in order to adapt to an ever-changing environment. Transcription factors coordinate these responses by engaging in different protein complexes and exerting both positive and negative effects. ABA INSENSITIVE 5 (ABI5) binding proteins (AFPs), which are closely related to NOVEL INTERACTOR OF JAZ (NINJA)-like proteins, are known for their fundamental role in plants’ morphological and physiological growth. Recent studies have shown that AFPs regulate several hormone-signalling pathways, including abscisic acid (ABA) and gibberellic acid (GA). Here, we review the genetic control of AFPs and their crosstalk with plant hormone signalling, and discuss the contributions of AFPs to plants’ growth and development."
Authors: Minglei Zhao, Chun-Lin Shi and Jianguo Li.
Fruit Research (2024)
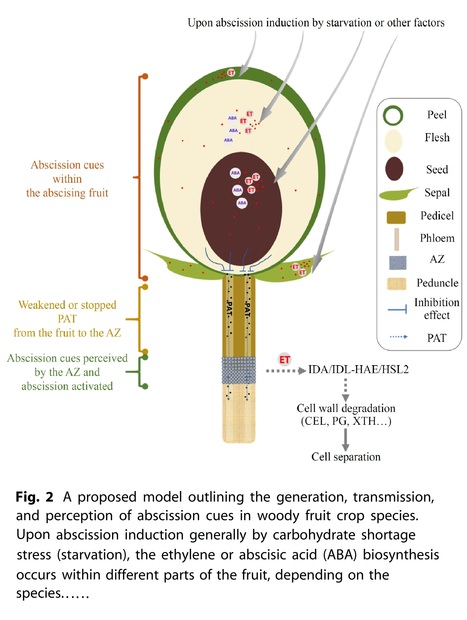
Abstract: "From an evolutionary perspective, fruit abscission is an intelligent regulatory mechanism by which fruit trees adapt to their environment and ensure offspring. However, from an agricultural production standpoint, unwanted fruit abscission can cause significant loss in fruit yield and economic value. Therefore, investigating the mechanisms of fruit abscission has always been an important focus in the field of plant research. Acquiring a thorough comprehension of the underlying mechanisms responsible for fruit abscission is highly valuable for enhancing fruit crop breeding and optimizing harvesting practices. In this review, we focus on fruit abscission, particularly discussing the nature of abscission cues within the abscising fruit, how these signals are generated and transmitted, and how the abscission zone cells perceive and respond to these signals in woody fruit crops."
Author: Xiufen Dong, Xianfeng Liu, Lina Cheng, Ruizhen Li, Siqi Ge, Sai Wang, Yue Cai, Yang Liu, Sida Meng, Cai-Zhong Jiang, Chun-Lin Shi, Tianlai Li, Daqi Fu, Mingfang Qi and Tao Xu.
Journal of Integrative Plant Biology (2024)
Abstract: "Auxin regulates flower and fruit abscission, but how developmental signals mediate auxin transport in abscission remains unclear. Here, we reveal the role of the transcription factor BEL1-LIKE HOMEODOMAIN11 (SlBEL11) in regulating auxin transport during abscission in tomato (Solanum lycopersicum). SlBEL11 is highly expressed in the fruit abscission zone, and its expression increases during fruit development. Knockdown of SlBEL11 expression by RNA interference (RNAi) caused premature fruit drop at the breaker (Br) and 3 d post-breaker (Br+3) stages of fruit development. Transcriptome and metabolome analysis of SlBEL11-RNAi lines revealed impaired flavonoid biosynthesis and decreased levels of most flavonoids, especially quercetin, which functions as an auxin transport inhibitor. This suggested that SlBEL11 prevents premature fruit abscission by modulating auxin efflux from fruits, which is crucial for the formation of an auxin response gradient. Indeed, quercetin treatment suppressed premature fruit drop in SlBEL11-RNAi plants. DNA affinity purification sequencing (DAP-seq) analysis indicated that SlBEL11 induced expression of the transcription factor gene SlMYB111 by directly binding to its promoter. Chromatin immunoprecipitation-quantitative polymerase chain reaction and electrophoretic mobility shift assay showed that S. lycopersicum MYELOBLASTOSIS VIRAL ONCOGENE HOMOLOG111 (SlMYB111) induces the expression of the core flavonoid biosynthesis genes SlCHS1, SlCHI, SlF3H, and SlFLS by directly binding to their promoters. Our findings suggest that the SlBEL11–SlMYB111 module modulates flavonoid biosynthesis to fine-tune auxin efflux from fruits and thus maintain an auxin response gradient in the pedicel, thereby preventing premature fruit drop."
Authors: Yuki Furuta, Haruka Yamamoto, Takeshi Hirakawa, Akira Uemura, Margaret Anne Pelayo, Hideaki Iimura, Naoya Katagiri, Noriko Takeda-Kamiya, Kie Kumaishi, Makoto Shirakawa, Sumie Ishiguro, Yasunori Ichihashi, Takamasa Suzuki, Tatsuaki Goh, Kiminori Toyooka, Toshiro Ito & Nobutoshi Yamaguchi
Nature Communications (2024)
Editor's view: In angiosperms, petal abscission is crucial for reproductive success and seed dispersion. However, the regulation of this abscission remains unclear. Here, the authors identify a process of petal abscission regulated by jasmonic acid via autophagy at the base of Arabidopsis petals.
Abstract: "In angiosperms, the transition from floral-organ maintenance to abscission determines reproductive success and seed dispersion. For petal abscission, cell-fate decisions specifically at the petal-cell base are more important than organ-level senescence or cell death in petals. However, how this transition is regulated remains unclear. Here, we identify a jasmonic acid (JA)-regulated chromatin-state switch at the base of Arabidopsis petals that directs local cell-fate determination via autophagy. During petal maintenance, co-repressors of JA signaling accumulate at the base of petals to block MYC activity, leading to lower levels of ROS. JA acts as an airborne signaling molecule transmitted from stamens to petals, accumulating primarily in petal bases to trigger chromatin remodeling. This allows MYC transcription factors to promote chromatin accessibility for downstream targets, including NAC DOMAIN-CONTAINING PROTEIN102 (ANAC102). ANAC102 accumulates specifically at the petal base prior to abscission and triggers ROS accumulation and cell death via AUTOPHAGY-RELATED GENEs induction. Developmentally induced autophagy at the petal base causes maturation, vacuolar delivery, and breakdown of autophagosomes for terminal cell differentiation. Dynamic changes in vesicles and cytoplasmic components in the vacuole occur in many plants, suggesting JA–NAC-mediated local cell-fate determination by autophagy may be conserved in angiosperms."
Authors: Agata Kućko, Juan de Dios Alché, Timothy John Tranbarger and Emilia Wilmowicz.
Journal of Plant Physiology (2023)
Highlights: • ABA and ET stimulate jasmonic acid accumulation in the flower abscission zone. • Manifestation of lipid metabolism initiation is the induction of LOX by ABA and ET. • COI1 is sensitive to hormonal stimulators of flower abscission. • ABA- and ET-mediated abscission of flowers is linked with MYC2 transcription factor. • ABA and ET act via jasmonates to promote flower abortion in lupine.
Abstract: "The appropriate timing of plant organ abscission determines plant growth, development, reproductive success, and yield in relation to crop species. Among these, yellow lupine is an example of a crop species that loses many fully developed flowers, which limits the formation of pods with high-protein seeds and affects its economic value. Lupine flower abscission, similarly to the separation of other organs, depends on a complex regulatory network functioning in the cells of the abscission zone (AZ). In the present study, genetic, biochemical, and cellular methods were used to highlight the complexity of the interactions among strong hormonal stimulators of abscission, including abscisic acid (ABA), ethylene, and jasmonates (JAs) precisely in the AZ cells, with all results supporting that the JA-related pathway has an important role in the phytohormonal cross-talk leading to flower abscission in yellow lupine. Based on obtained results, we conclude that ABA and ET have positive influence on JAs biosynthesis and signaling pathway in time-dependent manner. Both phytohormones changes lipoxygenase (LOX) gene expression, affects LOX protein abundance, and JA accumulation in AZ cells. We have also shown that the signaling pathway of JA is highly sensitive to ABA and ET, given the accumulation of COI1 receptor and MYC2 transcription factor in response to these phytohormones. The results presented provide novel information about the JAs-dependent separation of organs and provide insight and details about the phytohormone-related mechanisms of lupine flower abscission."
Authors: Marta Pujol and Jordi Garcia-Mas.
Journal of Experimental Botany (2023)
Abstract: "Fruit ripening is a complex and highly regulated process where tomato and strawberry have been the model species classically used for studying climacteric and non-climacteric fleshy fruit ripening types, respectively. Melon has emerged as an alternative ripening model because climacteric and non-climacteric cultivars exist, which makes it possible to dissect the regulation of ripening using a genetic approach. Several QTLs that regulate climacteric fruit ripening have been identified to date, and their combination in both climacteric and non-climacteric genetic backgrounds resulted in lines with different ripening behaviors, demonstrating that the climacteric intensity can be genetically modulated. The review discusses our current knowledge of the physiological changes observed during melon climacteric fruit ripening as ethylene production, fruit abscission, chlorophyll degradation, firmness and aroma, as well as their complex genetic control. From pioneer experiments in which ethylene biosynthesis was silenced, to the recent genetic edition of ripening regulators, current data suggest that the climacteric response is determined by the interaction of several loci under quantitative inheritance. The exploitation of the rich genetic diversity of melon will enable the discovery of additional genes involved in the regulation of the climacteric response, ultimately leading to breeding aromatic melon fruits with extended shelf life."
Authors: Pingyu Wang, Ting Wu, Chen Jiang, Baowen Huang and Zhengguo Li.
Plant Science (2023)
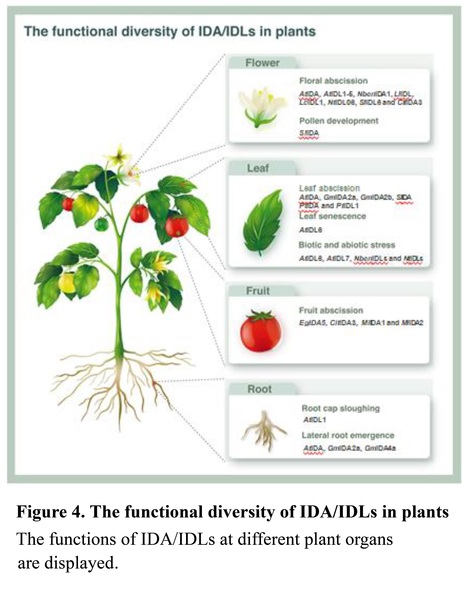
Highlights • The INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) peptide was first isolated by screening for floral abscission deficient mutants in Arabidopsis. The IDA/IDA-LIKE (IDLs) peptides are highly conserved in plants, as well as the signal transduction pathway. • The IDA/IDLs peptides are perceived by the receptor complex HAESA (HAE)/HAESA-LIKE1 (HSL1)/HAESA-LIKE2 (HSL2)-SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES (SERKs) and transduced through mitogen-activated protein kinase (MAPK) cascades, which induce specific transcription factors to modulate genes expression. • The IDA/IDLs peptides are key regulators in plant growth and development and stress responses.
Abstract: "As signal molecules, plant peptides play key roles in intercellular communication during growth and development, as well as stress responses. The 14-amino-acid (aa) INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) peptide was originally identified to play an essential role in the floral organ abscission of Arabidopsis. It is synthesized from its precursor, a small protein containing 77-aa residues with an N-terminal signal peptide sequence. Recently, the IDA/IDA-like (IDLs) genes are isolated in several angiosperms and are highly conserved in land plants. In addition, IDA/IDLs are not only involved in organ abscission but also in multiple biological processes, including biotic and abiotic stress response. Here, we summarize the post-translational modification and proteolytic processing, the evolutionary conservation, and the potential regulatory function of IDA/IDLs, and also present future perspectives to investigate the IDA/IDLs signaling pathway. We anticipate that this detailed knowledge will help to improve the understanding of the molecular mechanism of plant peptide signaling."
Authors: Estanis Torres and Luís Asín.
Journal of Plant Growth Regulation (2023)
Abstract: "The effect of temperature on the ability of 2-chloroethylphosphonic acid (ethephon, ETH) and the 1-aminocyclopropane carboxylic acid (ACC) to induce ethylene production in fruitlets and abscission of fruitlets and leaves when applied at postbloom (~15 mm fruit diameter) was studied using 5-year-old ‘Sweet Lady’/Rootpac-20 peach [Prunus persica (L.) Batsch] trees kept in pots in environment-controlled growth rooms. ETH at 150 mg L−1 and ACC at 500 mg L−1 effectively thinned peaches. Increasing the temperature from 10 to 20 °C pronounced the ETH-induced fruit and leaf abscission and the ACC-induced leaf abscission and decreased stomatal conductance in leaves. However, the ACC thinning effect was not affected by increasing temperature, as well as ETH- and ACC-induced ethylene production in fruitlets and in the quantum yield of PSII photochemistry. Changes in ethylene peak in fruitlets and stomatal conductance in leaves were related to the abscission response."
Authors: Hongmei Shu, Shangwen Sun, Xiaojing Wang, Changqin Yang, Guowei Zhang, Yali Meng, Youhua Wang, Wei Hu and Ruixian Liu.
International Journal of Molecular Sciences (2022)
Abstract: "Thidiazuron (TDZ) is the main defoliant used in production to promote leaf abscission for machine-picked cotton. Under low temperatures, the defoliation rate of cotton treated with TDZ decreases and the time of defoliation is delayed, but there is little information about this mechanism. In this study, RNA-seq and physiological analysis are performed to reveal the transcriptome profiling and change in endogenous phytohormones upon TDZ treatment in abscission zones (AZs) under different temperatures (daily mean temperatures: 25 °C and 15 °C). Genes differentially expressed in AZs between TDZ treatment and control under different temperatures were subjected to gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses to compare the enriched GO terms and KEGG pathways between the two temperature conditions. The results show that, compared with the corresponding control group, TDZ induces many differentially expressed genes (DEGs) in AZs, and the results of the GO and KEGG analyses show that the plant hormone signaling transduction pathway is significantly regulated by TDZ. However, under low temperature, TDZ induced less DEGs, and the enriched GO terms and KEGG pathways were different with those under normal temperature condition. Many genes in the plant hormone signal transduction pathway could not be induced by TDZ under low temperature conditions. In particular, the upregulated ethylene-signaling genes and downregulated auxin-signaling genes in AZs treated with TDZ were significantly affected by low temperatures. Furthermore, the expression of ethylene and auxin synthesis genes and their content in AZs treated with TDZ were also regulated by low temperature conditions. The upregulated cell wall hydrolase genes induced by TDZ were inhibited by low temperatures. However, the inhibition of low temperature on genes in AZs treated with TDZ was relieved with the extension of the treatment time. Together, these results indicate that the responses of ethylene and auxin synthesis and the signaling pathway to TDZ are inhibited by low temperatures, which could not induce the expression of cell wall hydrolase genes, and then inhibit the separation of AZ cells and the abscission of cotton leaves. This result provides new insights into the mechanism of defoliation induced by TDZ under low temperature conditions."
Authors: Chuyan Jiang, Yue Liang, Shuning Deng, Yang Liu, Haohao Zhao, Susu Li, Cai-Zhong Jiang, Junping Gao and Chao Ma.
New Phytologist (2023)
Abstract: "• In many plant species, petal abscission can be considered the final step of petal senescence. Cytokinins (CKs) are powerful suppressors of petal senescence; however, their role in petal abscission is ambiguous. • Here, we observed that, in rose (Rosa hybrida), biologically active CK is accumulated during petal abscission, and acts as an accelerator of the abscission. Using combination of reverse genetics, and molecular and biochemical techniques, we explored the roles of a LESION SIMULATING DISEASE1 (LSD1) family member RhLOL1 interacting with a bHLH transcription factor RhILR3 in CK-induced petal abscission. • Silencing RhLOL1 delays rose petal abscission, while the overexpression of its ortholog SlLOL1 in tomato (Solanum lycopersicum) promotes pedicel abscission, indicating the conserved function of LOL1 in activating plant floral organ abscission. In addition, we identify a bHLH transcription factor, RhILR3, that interacts with RhLOL1. We show that RhILR3 binds to the promoters of the auxin signaling repressor Aux/IAA genes to inhibit their expression; however, the interaction of RhLOL1 with RhILR3 activates the expression of the Aux/IAA genes including RhIAA4-1. Silencing RhIAA4-1 delays rose petal abscission. • Our results thus reveal a RhLOL1–RhILR3 regulatory module involved in CK-induced petal abscission via the regulation of the expression of the Aux/IAA genes."
Authors: C. Mesejo, A. Martínez-Fuentes, C. Reig and M. Agustí.
Scientia Horticulturae (2022)
Highlights: • ‘Orri’ mandarin has a low parthenocarpic ability, in accordance with its low CcGA3ox1 gene expression, but the application of GA3 does not increase fruit set. • Ringing branches performed 35-40 days after anthesis increases fruitlet growth rate. This effect correlates inversely with the fruitlet abscission rate, and positively with cell division, i.e., with the CcCYCA1.1 gene expression in the fruitlet, but not with the GA biosynthesis, i. e., with the CcGA3ox1 gene expression. • Ringing branches significantly increased CcPIN1 gene expression in the fruitlet. • Results suggest that ringing protects the fruitlet abscission zone mediated by polar auxin transport from the fruit, which allows the fruitlet to maintain carbohydrate assimilation and continue growing.
Abstract: "Ringing branches is a technique which is widely used to increase the yield of Citrus cultivars with low parthenocarpic ability. When performed during the physiological fruitlet abscission stage it prevents fruitlet drop and increases the number of fruits harvested. This effect has been related with an increased carbohydrate supply, which requires an enhanced photosynthesis efficiency of leafy flowering shoots. Since ringing also reduces vegetative growth, both the number of shoots and the leaves per shoot, the mechanism by which the carbohydrate supply is increased should be revised. Our results show that ringing carried out at this stage maintains the ability of the ovary for cell division mediated by the availability of carbohydrates, as indicated by an increased CcCYCA1.1 expression. But this effect is not linked with an increase in GA1 biosynthesis (CcGA3ox1 expression), as this occurs during fruit set; hence, hormones other than gibberellin must be controlling the physiological fruitlet abscission in response to ringing. We found that an increased expression of the auxin efflux carrier CcPIN1 gene suggests that ringing induces the auxin export out of the fruitlet and transport to the abscission zone (AZ-C), thus inhibiting its activation and allowing carbohydrates supply to the fruitlet which, thus, prevents abscission and continues growth."
Authors: Jian Wu, Huimin Liu, Sichao Ren, Panpan Li, Xue Li, Li Lin, Qinfu Sun, Long Zhang, Chen Lin and Youping Wang.
Journal of Experimental Botany (2022)
Excerpts: "To investigate the function of IDA on floral organ abscission, BnC06.IDA and BnA07.IDA were knocked out using CRISPR/Cas9 genome editing. To that end, two guide RNAs (gRNAs), gRNA1 and gRNA2, were designed to specifically target two regions of the coding sequence (Tgt1 and Tgt2) of BnC06.IDA and BnA07.IDA (Figure 1C). The CRISPR/Cas9 construct contained two gRNAs driven by Arabidopsis U3b and U3d promoters, and a Cas9 gene driven by the 35S promoter (Figure 1C)."
"Strikingly, the double mutants displayed a floral abscission-defective phenotype in which floral organs remained attached to developing siliques, and dry and colorless senesced floral parts remained attached to mature siliques (Figure 2, A and B). In contrast, the detached petals and stamens in wild-type and single mutants of bna07.ida and bnc06.ida adhered to leaves or petioles (Figure 2A)."
"In summary, our results demonstrate that BnA07.IDA and BnC06.IDA are functionally redundant and are involved in the regulation of floral abscission in OSR. We successfully generated OSR plants with abscission-defective floral organs by simultaneously inactivating two BnIDA genes using CRISPR/Cas9 (Figure 2G). The double mutant line, with its allele-specific markers, can be applied for breeding OSR cultivars with non-abscising floral organs that are anticipated to lengthen the flowering period for ornamental varieties, and to avoid Sclerotinia infection via abscised petals and stamens."
Authors: Xianfeng Liu, Lina Cheng, Ruizhen Li, Yue Cai, Xiaoyang Wang, Xin Fu, Xiufen Dong, Mingfang Qi, Cai-zhong Jiang, Tao Xu and Tianlai Li.
Plant Physiology (2022)
Abstract: "Plant organ abscission, a process that is important for development and reproductive success, is inhibited by the phytohormone auxin and promoted by another phytohormone, jasmonic acid (JA). However, the molecular mechanisms underlying the antagonistic effects of auxin and JA in organ abscission are unknown. We identified a tomato (Solanum lycopersicum) class III homeodomain-leucine zipper transcription factor, HOMEOBOX15A (SlHB15A), which was highly expressed in the flower pedicel abscission zone and induced by auxin. Knocking out SlHB15A using clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 technology significantly accelerated abscission. In contrast, overexpression of microRNA166-resistant SlHB15A (mSlHB15A) delayed abscission. RNA sequencing and reverse transcription quantitative PCR analyses showed that knocking out SlHB15A altered the expression of genes related to JA biosynthesis and signaling. Furthermore, functional analysis indicated that SlHB15A regulates abscission by depressing JA-isoleucine (JA-Ile) levels through inhabiting the expression of JASMONATE-RESISTANT1 (SlJAR1), a gene involved in JA-Ile biosynthesis, which could induce abscission-dependent and -independent ethylene signaling. SlHB15A bound directly to the SlJAR1 promoter to silence SlJAR1, thus delaying abscission. We also found that flower removal enhanced JA-Ile content and that application of JA-Ile severely impaired the inhibitory effects of auxin on abscission. These results indicated that SlHB15A mediates the antagonistic effect of auxin and JA-Ile during tomato pedicel abscission, while auxin inhibits abscission through the SlHB15A-SlJAR1 module."
|
Authors: Xingshuai Ma, Zidi He, Ye Yuan, Zhijian Liang, Hang Zhang, Vilde Olsson Lalun, Zhuoyi Liu, Yanqing Zhang, Zhiqiang Huang, Yulian Huang, Jianguo Li and Minglei Zhao.
Journal of Integrative Plant Biology (2024)
Abstract: "At the physiological level, the interplay between auxin and ethylene has long been recognized as crucial for the regulation of organ abscission in plants. However, the underlying molecular mechanisms remain unknown. Here, we identified transcription factors involved in indoleacetic acid (IAA) and ethylene (ET) signaling that directly regulate the expression of INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) and its receptor HAESA (HAE), which are key components initiating abscission. Specifically, litchi IDA-like 1 (LcIDL1) interacts with the receptor HAESA-like 2 (LcHSL2). Through in vitro and in vivo experiments, we determined that the auxin response factor LcARF5 directly binds and activates both LcIDL1 and LcHSL2. Furthermore, we found that the ETHYLENE INSENSITIVE 3-like transcription factor LcEIL3 directly binds and activates LcIDL1. The expression of IDA and HSL2 homologs was enhanced in LcARF5 and LcEIL3 transgenic Arabidopsis plants, but reduced in ein3 eil1 mutants. Consistently, the expressions of LcIDL1 and LcHSL2 were significantly decreased in LcARF5- and LcEIL3-silenced fruitlet abscission zones (FAZ), which correlated with a lower rate of fruitlet abscission. Depletion of auxin led to an increase in 1-aminocyclopropane-1-carboxylic acid (the precursor of ethylene) levels in the litchi FAZ, followed by abscission activation. Throughout this process, LcARF5 and LcEIL3 were induced in the FAZ. Collectively, our findings suggest that the molecular interactions between litchi AUXIN RESPONSE FACTOR 5 (LcARF5)–LcIDL1/LcHSL2 and LcEIL3–LcIDL1 signaling modules play a role in regulating fruitlet abscission in litchi and provide a long-sought mechanistic explanation for how the interplay between auxin and ethylene is translated into the molecular events that initiate abscission."
Authors: Jing Chen, Senlin Jiang, Guobin Yang, Lujun Li, Jing Li and Fengjuan Yang.
Plant Physiology and Biochemistry (2024)
Highlights: • SmMYB113 can increase flower drop rate in eggplant. • SmMYB113 regulates ethylene-dependent flower abscission by directly activating the ethylene biosynthetic genes. • SmERF38 enhanced the activation of SmMYB113 on the promoters of SmACS1 by interaction.
Abstract: "Flower abscission is an important developmental process that can significantly reduce the yield of horticultural plants. We previously reported that SmMYB113 is a key transcription factor promoting anthocyanin biosynthesis and improve fruit quality. However, the overexpression of SmMYB113 in eggplant increased flower drop rate and reduced fruit yield. Here, we elucidate the regulatory mechanisms of SmMYB113 on flower abscission in eggplant. RNA-seq analysis indicated that the regulation of flower abscission by SmMYB113 was associated with altered expression of genes related to ethylene biosynthesis and signal transduction, including ethylene biosynthetic genes SmACS1, SmACS8 and SmACO4. Then, the ethylene content in flowers and the function of ethephon (ETH, which promotes fruit ripening) and 1-Methylcyclopropene (1-MCP, which acts as an ethylene perception inhibitor) were analyzed, which revealed that SmMYB113 directly regulates ethylene-dependent flower abscission. Yeast one-hybrid and dual-luciferase assays revealed that SmMYB113 could directly bind to the promoters of SmACS1, SmACS8, and SmACO4 to activate their expression. Through construction of a yeast two-hybrid (Y2H) screening library, the protein SmERF38 was found to interact with SmMYB113, and verified by Y2H, bimolecular fluorescence complementation (BiFC), and luciferase complementation assay. Furthermore, dual-luciferase assays showed that SmERF38 enhanced the role of SmMYB113 on the promoters of SmACS1. Our results provided new insight into the molecular mechanism of flower abscission in eggplant."
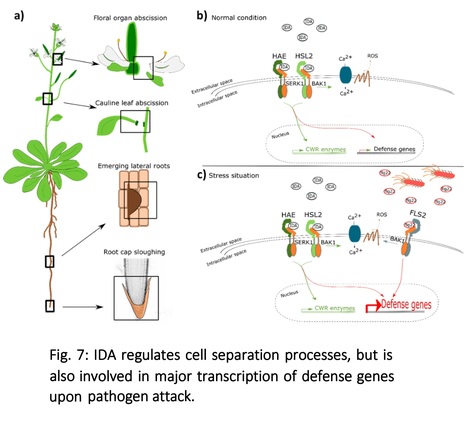
Authors: Vilde Olsson Lalun, Maike Breiden, Sergio Galindo-Trigo, Elwira Smakowska-Luzan, Rüdiger Simon and Melinka A. Butenko.
bioRxiv (2024)
Abstract: "The abscission of floral organs and emergence of lateral roots in Arabidopsis is regulated by the peptide ligand INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) and the receptor protein kinases HAESA (HAE) and HAESA-LIKE 2 (HSL2). During these cell separation processes, the plant induces defense-associated genes to protect against pathogen invasion. However, the molecular coordination between abscission and immunity has not been thoroughly explored. Here we show that IDA induces a release of cytosolic calcium ions (Ca2+) and apoplastic production of reactive oxygen species, which are signatures of early defense responses. In addition, we find that IDA promotes late defense responses by the transcriptional upregulation of genes known to be involved in immunity. When comparing the IDA induced early immune responses to known immune responses, such as those elicited by flagellin22 treatment, we observe both similarities and differences. We propose a molecular mechanism by which IDA promotes signatures of an immune response in cells destined for separation to guard them from pathogen attack."
Authors: Maarten Houben, John Vaughan-Hirsch, Jolien Pattyn, Wangshu Mou, Stijn Roden, Albert Roig Martinez, Elif Kabak, Savio Rodrigues, Asia Polko, Barbara De Coninck, Joseph J Kieber, Arnout R.D. Voet and Bram Van de Poel.
bioRxiv (2024)
Abstract: "Ethylene is involved in several developmental processes and responses towards (a)biotic stress. In seed plants, ethylene is produced from its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) by the enzyme ACC-oxidase (ACO). Despite its key role in ethylene synthesis, the ACO gene family has not yet been fully characterized. We investigated the five ACO members of Arabidopsis thaliana and revealed a tissue- and developmentally specific expression pattern. Furthermore, the five ACO enzymes each have a different capacity to produce ethylene. Combined, this allows for a precise spatial and temporal regulation of ethylene synthesis. At the sub-cellular level, we uncovered that ACOs reside in the cytosol, where ethylene is likely synthesized, but surprisingly also in the nucleus. Using reverse genetics of single and higher order aco mutants we revealed a high degree of gene redundancy and minimal phenotypes. A lack of ethylene synthesis by knocking out all five ACOs did not impair normal vegetative and generative development but did influence processes associated with high rates of ethylene production such as petal abscission. This suggests that ethylene is not a prime regulator of development, but more a moderator. We also showed that the inability to synthesize ethylene impairs some abiotic (nutrient deficiency and metal toxicity) and biotic (Botrytis cinerea) stress responses, similar as plants insensitive towards ethylene, corroborating the role of ethylene in mediating stress responses. In conclusion, the ACO gene family enables plants to fine-tune their ethylene synthesis rates, but a lack their off is not crucial for normal development and stress survival."
Authors: Hao Wu, Qi He, Bing He, Shuyi He, Longjun Zeng, Longbo Yang, Hong Zhang, Zhaoran Wei, Xingming Hu, Jiang Hu, Yong Zhang, Lianguang Shang, Suikang Wang, Peng Cui, Guosheng Xiong, Qian Qian and Quan Wang.
The Plant Cell (2023)
Abstract: "The elimination of seed shattering was a key step in rice (Oryza sativa) domestication. In this paper, we show that increasing the gibberellic acid (GA) content or response in the abscission region enhanced seed shattering in rice. We demonstrate that SLENDER RICE1 (SLR1), the key repressor of GA signaling, could physically interact with the rice seed shattering-related transcription factors QTL of seed shattering on chromosome 1 (qSH1), Oryza sativa HOMEOBOX 15 (OSH15), and SUPERNUMERARY BRACT GENE (SNB). Importantly, these physical interactions interfered with the direct binding of these three regulators to the lignin biosynthesis gene 4-COUMARATE:COENZYME A LIGASE 3 (4CL3), thereby de-repressing its expression. De-repression of 4CL3 led to increased lignin deposition in the abscission region, causing reduced rice seed shattering. Importantly, we also show that modulating GA content could alter the degree of seed shattering to increase harvest efficiency. Our results reveal that the “Green Revolution” phytohormone GA is important for regulating rice seed shattering, and we provide an applicable breeding strategy for high-efficiency rice harvesting."
Authors: Priya Singh, Shiv Kumar Maurya, Deepika Singh and Aniruddha P. Sane.
Plant Cell Reports (2023)
Key message: RbIDL1 and RbIDL4 are up-regulated in an ethylene-responsive manner during rose petal abscission and restored the Arabidopsis ida-2 mutant abscission defect suggesting functional conservation of the IDA pathway in rose.
Abstract: "Abscission is an ethylene-regulated developmental process wherein plants shed unwanted organs in a controlled manner. The INFLORESCENCE DEFICIENT IN ABSCISSION family has been identified as a key regulator of abscission in Arabidopsis, encoding peptides that interact with receptor-like kinases to activate abscission. Loss of function ida mutants show abscission deficiency in Arabidopsis. Functional conservation of the IDA pathway in other plant abscission processes is a matter of interest given the discovery of these genes in several plants. We have identified four members of the INFLORESCENCE DEFICIENT IN ABSCISSION-LIKE family from the ethylene-sensitive, early-abscising fragrant rose, Rosa bourboniana. All four are conserved in sequence and possess well-defined PIP, mIDa and EPIP motifs. Three of these, RbIDL1, RbIDL2 and RbIDL4 show a three–fourfold increase in transcript levels in petal abscission zones (AZ) during ethylene-induced petal abscission as well as natural abscission. The genes are also expressed in other floral tissues but respond differently to ethylene in these tissues. RbIDL1 and RbIDL4, the more prominently expressed IDL genes in rose, can complement the abscission defect of the Arabidopsis ida-2 mutant; while, promoters of both genes can drive AZ-specific expression in an ethylene-responsive manner even in Arabidopsis silique AZs indicating recognition of AZ-specific and ethylene-responsive cis elements in their promoters by the abscission machinery of rose as well as Arabidopsis.
Authors: Kaikai Zhu and Yajin Ye.
Plant Physiology (2023)
Excerpts: "In this issue of Plant Physiology, Wang et al. (2023) discovered a crosstalk between ABA signaling and the circadian clock in rice (Oryza sativa). This crosstalk module includes ABA receptor family member Regulatory Components of ABA Receptor 10 (RCAR10), ABA signaling pathway transcription factor ABA Insensitive 5 (ABI5), and clock component Pseudo-Response Regulator 95 (PRR95). Their results demonstrated that RCAR10-ABI5-PRR95 functions in a feedback loop to modulate ABA signaling and thus fine-tune seed germination and seeding growth (Wang et al., 2023)."
"In summary, the work presented here advances our understanding of the feedback regulation loop between ABA signaling and the circadian clock during seed germination, which is achieved through the RCAR10-ABI5-PRR95 regulatory module (Figure 1)."
Authors: Jianyang Liu, Md Tabibul Islam and Sherif M. Sherif.
Horticulturae (2022)
Abstract: "Preharvest fruit drop is a significant physiological problem that affects numerous commercially significant apple varieties, including ‘Gala.’ AVG and 1-MCP are two plant growth regulators commonly used to reduce fruit drop by reducing ethylene synthesis and perception, respectively. To optimize yield and market acceptance, a complete investigation of AVG and 1-MCP impacts on fruit drop and fruit quality of ‘Gala’ apples is required. In this study, four trials were conducted over the course of three years to determine the effects of AVG and 1-MCP on fruit drop and quality at harvest and after cold storage. Our results indicated that applications of AVG at the full-rate (130 mgL−1) three weeks before harvest (WBAH) were more effective at minimizing fruit drop than applications at the half-rate (65 mgL−1) and did not differ significantly from the double rate (260 mgL−1). Additionally, a single application of AVG was as effective in preventing fruit drop as two applications of 1-MCP. We also demonstrated that AVG decreased fruit skin pigmentation when used alone or in conjunction with GA4+7 or 1-MCP, while 1-MCP applications had no negative effect on fruit color. Finally, our data showed that when compared to 1-MCP and GA4+7, AVG alone was more effective in preventing stem-end splitting in Gala apples."
Authors: Shilun Gao, Yuan Gao, Ying Yang, Liming Jia and Xuehuang Weng.
HortScience (2022)
Abstract: "Nutrient deficiency leads to a high fruit abscission rate and low yields of Sapindus mukorossi Gaertn. (Soapberry), which is one of the most widely cultivated biodiesel feedstock forests in China. Exogenous sucrose can provide a solution to nutrient deficiency and fruit abscission leading to low yields; therefore, it was applied to whole trees at two stages, 20 days before blooming (DBB stage) and before fruit abscission [days before fruit abscission (DBFA) stage]. Six sucrose concentrations, 0%, 1%, 1.5%, 3%, 5%, and 7%, were sprayed three times using a completely randomized block design with five replications and six treatments. 13CO2 labeling experiments were performed after the three sprayings. The results indicated that the 3% treatment had the highest yield, reaching 15.9 kg/tree. During the DBB stage, the 3% treatment significantly increased the inflorescence fructose and glucose contents 1- to 1.2-times and resulted in the highest fruit gibberellic acid, leaf indole acetic acid (IAA), fruit IAA, and fruit zeatin contents; however, it decreased the inflorescence abscisic acid (ABA) from 16 μg/g to 4 μg/g. The 1.5% and 3% treatments significantly increased the carbohydrate content and decreased the fruit ABA content to 30% to 50% of the control level during the DBFA stage. High-concentration sugar treatment (>3%) increased the nitrogen, phosphorus, and potassium contents, which decreased the calcium and magnesium contents. The 13C-dispatching ability of the inflorescence was three-times greater than that of leaves under the high-concentration sugar treatment during the DBB stage. Supplying 1.5% sucrose nearly doubled the allocation capacity during the DBFA stage. The source-sink nutrient migration pathway showed that leaf and fruit sugars were directly correlated with phosphorus. Fruit fructose and glucose contents affected the leaf mineral element contents."
Authors: Gloria Serrano-Bueno, Pedro de los Reyes, Andrea Chini, Gabriel Ferreras-Garrucho, Víctor Sánchez de Medina-Hernández, Marta Boter, Roberto Solano and Federico Valverde.
Molecular Plant (2022)
Abstract: "In Arabidopsis, photoperiodic flowering is controlled by the hub gene CONSTANS (CO), while floral organ senescence is regulated by the jasmonates (JAs). As both processes are chronologically ordered, it remains unknown whether there are common regulators of both processes. In this study, we discovered that CO protein is accumulated in Arabidopsis flowers after the floral induction, whereas it displays a diurnal pattern in floral organs different from that in the leaves. We observed that altered CO expression could affect flower senescence and abscission by interfering with JA response, as shown by petal-specific transcriptomic analysis as well as CO overexpression in JA synthesis and signaling mutants. Interestingly, we found that CO has a ZIM-like domain that can mediate its interaction with the JA response repressor JAZ3 (jasmonate ZIM-domain 3). Their interaction could inhibit the repressor activity of JAZ3, resulting in the activation of downstream transcription factors involved in promoting flower senescence. Furthermore, we showed that CO, JAZ3 and the E3 ubiquitin ligase Coronatine Insensitive 1 (COI1) could form a protein complex in planta, which promotes the degradation of both CO and JAZ3 in the presence of JAs. Taken together, our results indicate that CO, a key regulator of photoperiodic flowering, is also involved in promoting flower senescence and abscission by augmenting JA signaling and response. We propose that the coordinated recruitment of photoperiodic and JA signaling pathways could be an efficient way for plants to chronologically order the floral process and ensure the success of offspring production."
Authors: Lina Cheng, Ruizhen Li, Xiaoyang Wang, Siqi Ge, Sai Wang, Xianfeng Liu, Jing He, Cai-Zhong Jiang, Mingfang Qi, Tao Xu and Tianlai Li.
The Plant Cell (2022)
Abstract: "Premature abscission of flowers and fruits triggered by low light stress can severely reduce crop yields. However, the underlying molecular mechanism of this organ abscission is not fully understood. Here, we show that a gene (SlCLV3) encoding CLAVATA3 (CLV3), a peptide hormone that regulates stem cell fate in meristems, is highly expressed in the pedicel abscission zone (AZ) in response to low light in tomato (Solanum lycopersicum). SlCLV3 knockdown and knockout lines exhibit delayed low light–induced flower drop. The receptor kinases SlCLV1 and BARELY ANY MERISTEM1 (SlBAM1) function in the SlCLV3 peptide–induced low light response in the AZ to decrease expression of the transcription factor gene WUSCHEL (SlWUS). DNA affinity purification sequencing identified the transcription factor genes KNOX-LIKE HOMEDOMAIN PROTEIN1 (SlKD1) and FRUITFULL2 (SlFUL2) as SlWUS target genes. Our data reveal that low light reduces SlWUS expression, resulting in higher SlKD1 and SlFUL2 expression in the AZ, thereby perturbing the auxin response gradient and causing increased ethylene production, eventually leading to the initiation of abscission. These results demonstrate that the SlCLV3-SlWUS signaling pathway plays a central role in low light–induced abscission by affecting auxin and ethylene homeostasis."
Authors: Wei-Han Chen, Pei-Tzu Lin, Wei-Han Hsu, Hsing-Fun Hsu, Ya-Chun Li, Chin-Wei Tsao, Mao-Cheng Hsu, Wan-Ting Mao and Chang-Hsien Yang
Communications Biology (2022)
Abstract: "FOREVER YOUNG FLOWER (FYF) has been reported to play an important role in regulating flower senescence/abscission. Here, we functionally analyzed five Arabidopsis FYF-like genes, two in the FYF subgroup (FYL1/AGL71 and FYL2/AGL72) and three in the SOC1 subgroup (SOC1/AGL20, AGL19, and AGL14/XAL2), and showed their involvement in the regulation of flower senescence and/or abscission. We demonstrated that in FYF subgroup, FYF has both functions in suppressing flower senescence and abscission, FYL1 only suppresses flower abscission and FYL2 has been converted as an activator to promote flower senescence. In SOC1 subgroup, AGL19/AGL14/SOC1 have only one function in suppressing flower senescence. We also found that FYF-like proteins can form heterotetrameric complexes with different combinations of A/E functional proteins (such as AGL6 and SEP1) and AGL15/18-like proteins to perform their functions. These findings greatly expand the current knowledge behind the multifunctional evolution of FYF-like genes and uncover their regulatory network in plants. Functional characterization of the FYF-like genes in Arabidopsis demonstrates roles in flower senescence/abscission and finds the FYF-associated heterotetrameric complexes in regulating flower abscission."
|



 Your new post is loading...
Your new post is loading...