Your new post is loading...
 Your new post is loading...
Authors: Ishfaq Majid Hurrah, Amit Kumar and Nazia Abbas.
Planta (2024)
Main conclusion: Overexpression of Artemisia annua jasmonic acid carboxyl methyltransferase (AaJMT) leads to enhanced artemisinin content in Artemisia annua.
Abstract: "Artemisinin-based combination therapies remain the sole deterrent against deadly disease malaria and Artemisia annua remains the only natural producer of artemisinin. In this study, the 1101 bp gene S-adenosyl-l-methionine (SAM): Artemisia annua jasmonic acid carboxyl methyltransferase (AaJMT), was characterised from A. annua, which converts jasmonic acid (JA) to methyl jasmonate (MeJA). From phylogenetic analysis, we confirmed that AaJMT shares a common ancestor with Arabidopsis thaliana, Eutrema japonica and has a close homology with JMT of Camellia sinensis. Further, the Clustal Omega depicted that the conserved motif I, motif III and motif SSSS (serine) required to bind SAM and JA, respectively, are present in AaJMT. The relative expression of AaJMT was induced by wounding, MeJA and salicylic acid (SA) treatments. Additionally, we found that the recombinant AaJMT protein catalyses the synthesis of MeJA from JA with a Km value of 37.16 µM. Moreover, site-directed mutagenesis of serine-151 in motif SSSS to tyrosine, asparagine-10 to threonine and glutamine-25 to histidine abolished the enzyme activity of AaJMT, thus indicating their determining role in JA substrate binding. The GC–MS analysis validated that mutant proteins of AaJMT were unable to convert JA into MeJA. Finally, the artemisinin biosynthetic and trichome developmental genes were upregulated in AaJMT overexpression transgenic lines, which in turn increased the artemisinin content."
Authors: Vojtěch Schmidt, Roman Skokan, Thomas Depaepe, Katarina Kurtović, Samuel Haluška, Stanislav Vosolsobě, Roberta Vaculíková, Anthony Pil, Petre Ivanov Dobrev, Václav Motyka, Dominique Van Der Straeten and Jan Petrášek.
Nature Communications (2024)
Editor's view: Here, the authors show that the biosynthesis of many compounds in green algae preceded their recruitment in phytohormone signaling and metabolism in land plants.
Abstract: "The genomes of charophyte green algae, close relatives of land plants, typically do not show signs of developmental regulation by phytohormones. However, scattered reports of endogenous phytohormone production in these organisms exist. We performed a comprehensive analysis of multiple phytohormones in Viridiplantae, focusing mainly on charophytes. We show that auxin, salicylic acid, ethylene and tRNA-derived cytokinins including cis-zeatin are found ubiquitously in Viridiplantae. By contrast, land plants but not green algae contain the trans-zeatin type cytokinins as well as auxin and cytokinin conjugates. Charophytes occasionally produce jasmonates and abscisic acid, whereas the latter is detected consistently in land plants. Several phytohormones are excreted into the culture medium, including auxin by charophytes and cytokinins and salicylic acid by Viridiplantae in general. We note that the conservation of phytohormone biosynthesis and signaling pathways known from angiosperms does not match the capacity for phytohormone biosynthesis in Viridiplantae. Our phylogenetically guided analysis of established algal cultures provides an important insight into phytohormone biosynthesis and metabolism across Streptophyta. Genomic evidence dates the origins of most phytohormones to terrestrialization or later."
Authors: Guangwei Sun, Xuanhao Zhang, Yi Liu, Liguang Chai, Daisong Liu, Zhenguo Chen and Shiyou Lü.
Phyton (2024)
Abstract: "The Nicotiana genus, commonly known as tobacco, holds significant importance as a crucial economic crop. Confronted with an abundance of herbivorous insects that pose a substantial threat to yield, tobacco has developed a diverse and sophisticated array of mechanisms, establishing itself as a model of plant ecological defense. This review provides a concise overview of the current understanding of tobacco’s defense strategies against herbivores. Direct defenses, exemplified by its well-known tactic of secreting the alkaloid nicotine, serve as a potent toxin against a broad spectrum of herbivorous pests. Moreover, in response to herbivore attacks, tobacco enhances the discharge of volatile compounds, harnessing an indirect strategy that attracts the predators of the herbivores. The delicate balance between defense and growth leads to the initiation of most defense strategies only after a herbivore attack. Among plant hormones, notably jasmonic acid (JA), play central roles in coordinating these defense processes. JA signaling interacts with other plant hormone signaling pathways to facilitate the extensive transcriptional and metabolic adjustments in plants following herbivore assault. By shedding light on these ecological defense strategies, this review emphasizes not only tobacco’s remarkable adaptability in its natural habitat but also offers insights beneficial for enhancing the resilience of current crops."
Authors: Yuanyuan Wu, Ying Sun, Wanmin Wang, Zizhao Xie, Chenghang Zhan, Liang Jin and Junli Huang.
Plant Physiology and Biochemistry (2024)
Highlights: • OsJAZ10 negatively modulates rice drought stress tolerance. • OsJAZ10 physically interacts with OsMYC2, and inhibits OsMYC2 transcriptional activation on ABA- and JA-biosynthetic genes, thus repressing ABA and JA response. • OsJAZ10 inhibits the transmission of osmotic stress-elicited systematic electrical signals, which is in parallel to the hormone response.
Abstract: "Jasmonic acid (JA) plays crucial functions in plant stress response, and the synergistic interaction between JA and abscisic acid (ABA) signaling is implicated to help plants adapt to environmental challenges, whereas the underlying molecular mechanism still needs to be revealed. Here, we report that OsJAZ10, a repressor in the JA signaling, represses rice drought tolerance via inhibition of JA and ABA biosynthesis. Function loss of OsJAZ10 markedly enhances, while overexpression of OsJAZ10ΔJas reduces rice drought tolerance. The osjaz10 mutant is more sensitive to exogenous ABA and MeJA, and produces higher levels of ABA and JA after drought treatment, indicating OsJAZ10 represses the biosynthesis of these two hormones. Mechanistic study demonstrated that OsJAZ10 physically interacts with OsMYC2. Transient transcriptional regulation assays showed that OsMYC2 activates the expression of ABA-biosynthetic gene OsNCED2, JA-biosynthetic gene OsAOC, and drought-responsive genes OsRAB21 and OsLEA3, while OsJAZ10 prevents OsMYC2 transactivation of these genes. Further, electrophoretic mobility shift assay (EMSA) confirmed that OsMYC2 directly binds to the promoters of OsNCED2 and OsRAB21. Electrical activity has been proposed to activate JA biosynthesis. Interestingly, OsJAZ10 inhibits the propagation of osmotic stress-elicited systemic electrical signals, indicated by the significantly increased PEG-elicited slow wave potentials (SWPs) in osjaz10 mutant, which is in accordance with the elevated JA levels. Collectively, our findings establish that OsJAZ10 functions as a negative regulator in rice drought tolerance by repressing JA and ABA biosynthesis, and reveal an important mechanism that plant integrates electrical events with hormone signaling to enhance the adaption to environmental stress."
Authors: André Kessler and Michael B. Mueller.
Plant Signaling & Behavior (2024)
Abstract: "Plant induced responses to environmental stressors are increasingly studied in a behavioral ecology context. This is particularly true for plant induced responses to herbivory that mediate direct and indirect defenses, and tolerance. These seemingly adaptive alterations of plant defense phenotypes in the context of other environmental conditions have led to the discussion of such responses as intelligent behavior. Here we consider the concept of plant intelligence and some of its predictions for chemical information transfer in plant interaction with other organisms. Within this framework, the flow, perception, integration, and storage of environmental information are considered tunable dials that allow plants to respond adaptively to attacking herbivores while integrating past experiences and environmental cues that are predictive of future conditions. The predictive value of environmental information and the costs of acting on false information are important drivers of the evolution of plant responses to herbivory. We identify integrative priming of defense responses as a mechanism that allows plants to mitigate potential costs associated with acting on false information. The priming mechanisms provide short- and long-term memory that facilitates the integration of environmental cues without imposing significant costs. Finally, we discuss the ecological and evolutionary prediction of the plant intelligence hypothesis."
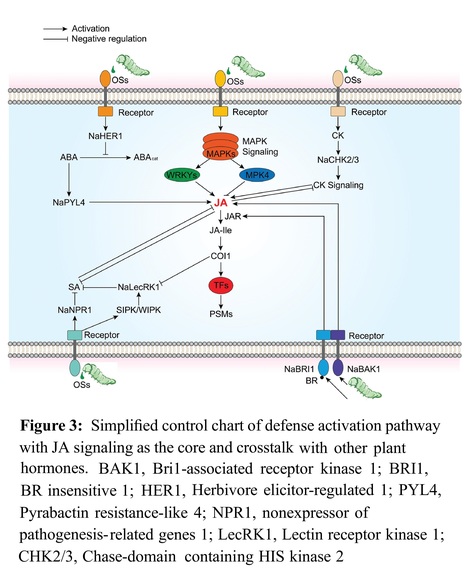
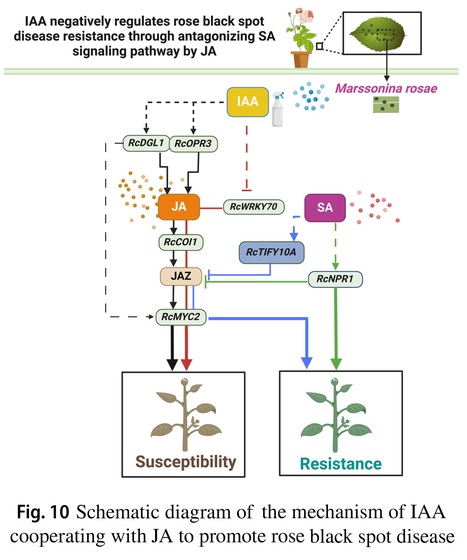
Authors: Tingliang Xu, Xiaowen Zheng, Yi Yang, Shumin Yang, Xingwan Yi, Chao Yu, Le Luo, Jia Wang, Tangren Cheng, Qixiang Zhang and Huitang Pan.
Planta (2024)
Main conclusion: IAA cooperates with JA to inhibit SA and negatively regulates rose black spot disease resistance.
Abstract: "Black spot disease caused by the fungus Marssonina rosae is the most prevalent and severe ailment in rose cultivation, leading to the appearance of black spots on leaves and eventual leaf fall, significantly impacting the utilization of roses in gardens. Salicylic acid (SA) and jasmonic acid (JA) are pivotal hormones that collaborate with indole-3 acetic acid (IAA) in regulating plant defense responses; however, the detailed mechanisms underlying the induction of black spot disease resistance by IAA, JA, and SA remain unclear. In this study, transcript analysis was conducted on resistant (R13–54) and susceptible (R12–26) lines following M. rosae infection. In addition, the impact of exogenous interference with IAA on SA- and JA-mediated disease resistance was examined. The continuous accumulation of JA, in synergy with IAA, inhibited activation of the SA signaling pathway in the early infection stage, thereby negatively regulating the induction of effective resistance to black spot disease. IAA administration alleviated the inhibition of SA on JA to negatively regulate the resistance of susceptible strains by further enhancing the synthesis and accumulation of JA. However, IAA did not contribute to the negative regulation of black spot resistance when high levels of JA were inhibited. Virus-induced gene silencing of RcTIFY10A, an inhibitor of the JA signaling pathway, further suggested that IAA upregulation led to a decrease in disease resistance, a phenomenon not observed when the JA signal was inhibited. Collectively, these findings indicate that the IAA-mediated negative regulation of black spot disease resistance relies on activation of the JA signaling pathway."
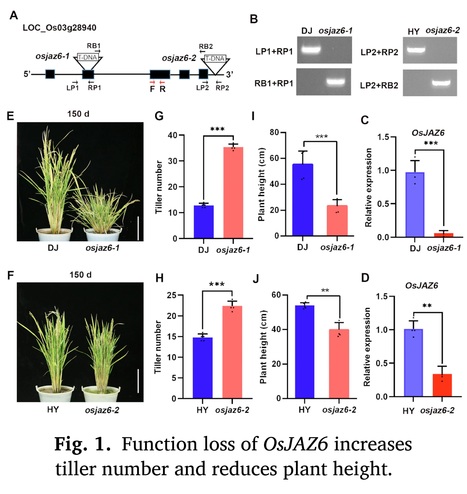
Authors: Wanmin Wang, Zizhao Xie, Yuanyuan Wu, Ying Sun, Chenghang Zhan, Liang Jin and Junli Huang.
Environmental and Experimental Botany (2024)
Highlights: • OsJAZ6 modulates rice tillering and drought response by integrating JA with GA signaling. • OsJAZ6 controls the tiller bud growth but not formation. • OsJAZ6 interacts with SLR1 to promote its degradation, which further destabilizes MOC1. • OsJAZ6 and SLR1 have opposite functions in regulating rice tiller bud growth and drought tolerance.
Abstract: "Jasmonic acid (JA) plays crucial functions during plant growth and stress response, but its roles and regulatory mechanism in plant branching remain largely unknown. Rice basal branching (tillering) is an essential agronomic trait that affects crop production. Here, we report that OsJAZ6, the repressor of JA signaling, negatively modulates rice tillering and drought stress tolerance. Loss-of-function mutants of OsJAZ6 exhibit a significant increase in tiller number, while OsJAZ6ΔJas-overexpression lines produce fewer tillers than wild-type plants. Further investigations show that function loss of OsJAZ6 promotes the tiller bud growth rather than formation. Mechanistic studies show that OsJAZ6 interacts with rice DELLA/SLR1 (SLENDER RICE 1), a transcription repressor of gibberellin (GA) signaling, and the interaction promotes SLR1 degradation, which further facilitates the degradation of rice tillering regulator MOC1 (MONOCULM 1), thereby inhibiting the tiller bud growth. In agreement, the slr1 mutant exhibits fewer tillers than wild type. Consistently, application of JA promotes the growth of tiller bud and thus increases the tiller number, while GA treatment results in opposite result. Meanwhile, osjaz6 mutants display enhanced drought tolerance, coupled with increased JA sensitivity, while the slr1 mutant shows the reverse behavior. Collectively, our data demonstrate that OsJAZ6 negatively modulates rice tillering as well as drought stress tolerance by destabilizing SLR1 protein. Our data shed light on the regulatory mechanism of controlling the tiller development and drought stress response in rice by the JA-OsJAZ6-SLR1 module."
Abstract: "Cold stress severely restricts growth and development, reduces yields, and impairs quality in tomatoes (Solanum lycopersicum). Amylase-associated starch degradation and soluble sugar accumulation have been implicated in adaptation and resistance to abiotic stress. Here, we report a β-amylase (BAM) gene, SlBAM3, which plays a central role in tomato cold tolerance. The expression of SlBAM3 was triggered by cold stress. SlBAM3 knockout using the CRISPR/Cas9 system retarded starch degradation and reduced soluble sugar accumulation in tomato plants, eventually attenuating cold tolerance. Expression analysis revealed that the SlBAM3 transcript level was boosted by MeJA. Furthermore, MYC2, an essential component of the JA signaling pathway, could bind to the SlBAM3 promoter and directly activate SlBAM3 transcription, as revealed by yeast one-hybrid and dual LUC assays. In addition, the suppression of MYC2 resulted in increased starch accumulation, decreased soluble sugar content, and reduced tolerance to cold stress in tomato plants. Taken together, these findings demonstrate that JA positively regulates β-amylase-associated starch degradation through the MYC2-SlBAM3 module in tomato during cold stress. The results of the present work expand our understanding of the mechanisms underlying BAM gene activation and starch catabolism under cold stress. The regulatory module of SlBAM3 can be further utilized to breed tomato cultivars with enhanced cold tolerance."
Authors: Xiaofang Li, Xin Zheng, Nikita Yadav, Shouvik Saha, El-Sayed Salama, Xiangkai Li, Likun Wang and Byong-Hun Jeon.
Plant Communications (2024)
Abstract: The Green Revolution of the mid-20th century transformed agriculture worldwide and has resulted in environmental challenges. A new approach, the Second Green Revolution, seeks to enhance agricultural productivity while minimizing negative environmental impacts. Plant microbiomes play critical roles in plant growth and stress responses, and understanding plant–microbiome interactions is essential for developing sustainable agricultural practices that meet food security and safety challenges, which are among the United Nations Sustainable Development Goals. This review provides a comprehensive exploration of key deterministic processes crucial for developing microbiome management strategies, including the host effect, the facilitator effect, and microbe–microbe interactions. A hierarchical framework for plant microbiome modulation is proposed to bridge the gap between basic research and agricultural applications. This framework emphasizes three levels of modulation: single-strain, synthetic community, and in situ microbiome modulation. Overall, rational management of plant microbiomes has wide-ranging applications in agriculture and can potentially be a core technology for the Second Green Revolution."
Authors: Wenyuan Ruan, Meina Guo and Keke Yi.
Molecular Plant (2024)
Excerpts: "A recent study by Dr. Hong's group (He et al, 2024) has shed new light on the roles of PHRs and SPXs in modulating the trade-off between growth and immunity in response to Pi availability in rice. They elegantly demonstrated that the OsPHR2-OsSPX1/2 module switches JA and BR signaling outputs in response to Pi availability. Under Pi-sufficient conditions, OsSPX1/2 inhibits OsPHR2 from activating JA-mediated defense. Meanwhile, OsBZR1 confers basal resistance by regulating sakuranetin biosynthesis. Under Pi starvation conditions, OsPHR2 dissociates from the OsPHR2-OsSPX1/2 complex and turns on JA signaling and sakuranetin accumulation to suppress Magnaporthe oryzae growth; meanwhile, the released OsSPX1/2 binds to OsBZR1 to suppress BR signaling and plant growth."
"The findings by Hong's group not only highlight the functional diversity of the central Pi signaling regulators but also underscore their crucial role in modulating the trade-off between growth and immunity in plants. These regulators extend beyond their traditional function in Pi signaling and homeostasis, playing a critical role in regulating the interactions between plants and various biotic species, including both harmful and beneficial organisms such as bacteria, fungi, and insects (Figure 1)."
Authors: Zi-Wei Yan, Jie Liu, Hui-Cheng Chen, Mu-Yang Wang, Wen-Juan Cai, Jian-Xu Li, Jia-Wei Wang and Ying-Bo Mao.
bioRxiv (2024)
Abstract: "Wounding is a common stress experienced by plants, resulting in a quick elicitation of jasmonates (JAs). The widely accepted mechanism of COI1-JAZ coreceptor posits that COI1 directly interacts with the active ligand of JAs, whereas JAZs facilitate to trap the ligand in the binding pocket. Here we show that the multiple JAZs of Arabidopsis undergo phase separation in response to wounding. Taking JAZ1 as an example, we found it is an independent sensor of JA-Ile, the active ligand in most plants. Under basal condition, dispersed JAZ1 interacts with MYC2 to inhibit downstream gene expressions. Upon wounding, JAZ1 binds to JA-Ile and forms condensates, reducing its affinity with MYC2 and tending to recruit COI1 as well as other proteasome related components for quick degradation, thereby accelerating signaling output. Our investigation unveils the underlying mechanism by which JAZ1 regulates swift signaling transduction via phase separation upon the direct perception of JA-Ile."
Authors: Jian Che, Xu Li and Yidan Ouyang.
Plant Communications (2024)
Excerpts: "The use of strong heterosis from indica-japonica hybrid rice could greatly increase yield potential in the future. Nevertheless, due to their long-term adaptation to different environments, indica and japonica rice varieties have evolved distinct phenotypic variations from growth to flowering. This leads to the existence of multiple reproductive barriers between the two subspecies. Consequently, prezygotic reproductive isolation caused by differentiated DFOTs and postzygotic reproductive isolation, such as hybrid sterility, which occurs in intersubspecific crosses, hinder the exploitation of indica-japonica hybrid rice (Figure 1)."
"Another possible approach for optimizing non-overlapping DFOTs might be the spraying of exogenous plant hormones (Wang et al., 2023). For example, spraying methyl jasmonate (MeJA) shows extensive applicability in regulating DFOT. In fact, OsMYB8 can regulate floret opening by inducing the accumulation of endogenous JA-Ile in lodicules. Latest research also confirmed the significance of JA in floret opening process, as overexpressing the JA biosynthesis gene OsOPR7 and knocking out the JA inactivation gene OsHAN1 can effectively promote the DFOT of japonica rice (Wang et al., 2024). Advanced DFOT can also be achieved by knockout of the JA signal suppressor genes OsJAZ7 and OsJAZ9. Other hormones, such as auxins and gibberellins, may also be involved in DFOT regulation and have the potential to improve DFOT."
Authors: Khansa Mekkaoui, Ranjit Baral, Fiona Smith, Moritz Klein, Ivo Feussner and Bettina Hause.
bioRxiv (2024)
Abstract: "In addition to jasmonoyl-isoleucine (JA-Ile), a well-established signaling molecule for plant growth and defense, its precursor, cis-12-oxo-phytodienoic acid (OPDA), is thought to possess independent signaling functions. Its perception in vascular plants is still uncharacterized. Several OPDA functions in Arabidopsis were inferred from a mutant that is affected in the function of the OPDA REDUCTASE3 (OPR3), catalyzing the conversion of OPDA within peroxisomes. Recently, opr3 plants were found to accumulate JA-Ile via a cytosolic OPR2-mediated bypass. Given the uncoupling of OPDA and JA biosynthesis in the JA-deficient mutant opr2opr3, potential OPDA signaling was investigated by a transcriptome approach comparing wild type, opr2opr3 and the JA- and OPDA-deficient mutant allene oxide synthase. Dissecting the wound response of seedlings revealed that OPDA lacked a transcriptional signature, and that previously characterized OPDA-response genes were wound-induced independently of OPDA. Exogenous application of OPDA to opr2opr3 seedlings led to JA-Ile formation and signaling even in absence of OPR2 and OPR3 and resulted in activation of sulfur assimilation. These divergent responses to endogenously synthesized and applied OPDA suggest a compartmentalization of endogenous OPDA which was investigated by a trans-organellar complementation approach. OPR3 complemented the opr2opr3 mutant in terms of fertility and wound-induced JA-Ile production irrespective of its subcellular localization. In vitro enzymatic activity of OPR3, however, showed conversion of OPDA and 4,5-didehydro-JA (4,5-ddh-JA), therefore not allowing to conclude which compound is translocated. Dissecting the conversion of either OPDA or 4,5-ddh-JA by OPR2 and OPR1 organelle variants pointed to a strong OPDA compartmentalization supporting its lacking signaling capacity."
|
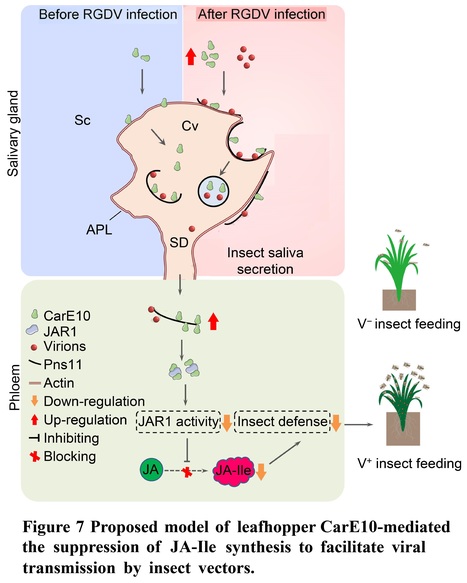
Authors: Yunhua Chi, Hongxiang Zhang, Siyu Chen, Yu Cheng, Xiaofeng Zhang, Dongsheng Jia, Qian Chen, Hongyan Chen and Taiyun Wei.
Plant Communications (2024)
Abstract: "Plant jasmonoyl-L-isoleucine (JA-Ile) is a major defense signal against insect feeding, but whether or how insect salivary effectors suppress JA-Ile synthesis and thus facilitate viral transmission in plant phloem remains elusive. Insect carboxylesterases (CarEs) are the third major family of detoxification enzymes. Here, we identify a new leafhopper CarE10 that specifically expressed in salivary glands and is secreted into rice phloem as the saliva component. Leafhopper CarE10 directly binds and promotes rice Jasmonate resistant 1 (JAR1) degradation by the proteasome system. Moreover, the direct association of CarE10 with JAR1 obviously impairs JAR1 enzyme activity for JA conversion to JA-Ile in in-vitro JA-Ile synthesis system. A devastating rice reovirus activates and promotes co-secretion of virions and CarE10 by virus-induced vesicles into saliva-stored salivary cavities of leafhopper vectors and ultimately into rice phloem to establish initial infection. Furthermore, virus-mediated increase of CarE10 secretion or overexpression of CarE10 in transgenic rice plants causes the reduced levels of JAR1 and thus suppresses JA-Ile synthesis, thereby promoting host attractiveness to insect vectors and facilitating initial viral transmission. Our findings provide insights into how insect salivary protein CarE10 suppresses host JA-Ile synthesis to benefit initial virus transmission in rice phloem."
Authors: Kun Dong, Fuqing Wu, Siqi Cheng, Shuai Li, Feng Zhang, Xinxin Xing, Xin Jin, Sheng Luo, Miao Feng, Rong Miao, Yanqi Chang, Shuang Zhang, Xiaoman You, Peiran Wang, Xin Zhang, Cailin Lei, Yulong Ren, Shanshan Zhu, Xiuping Guo, Chuanyin Wu, Dong-Lei Yang, Qibing Lin, Zhijun Cheng and Jianmin Wan.
Molecular Plant (2024)
Abstract: "Although both protein arginine methylation (PRMT) and jasmonate (JA) signaling are crucial for regulating plant development, the relationship between these processes in spikelet development control remains unclear. Here, we utilized CRISPR/Cas9 technology to generate two OsPRMT6a loss-of-function mutants exhibiting various abnormal spikelet structures. Additionally, we found that OsPRMT6a could methylate arginine residues in the JA signal repressors OsJAZ1 and OsJAZ7. Arginine methylation of OsJAZ1 increased the affinity of OsJAZ1 for the JA receptors OsCOI1a and OsCOI1b in the presence of jasmonates (JAs), subsequently promoting the ubiquitination of OsJAZ1 by the SCFOsCOI1a/OsCOI1b complex and degradation via the 26S proteasome. This process ultimately released OsMYC2, a core transcriptional regulator in the JA signaling pathway, to activate or repress JA-responsive genes, thereby maintaining normal plant (spikelet) development. However, in the osprmt6a-1 mutant, reduced arginine methylation of OsJAZ1 impaired the interaction between OsJAZ1 and OsCOI1a/OsCOI1b in the presence of JAs. As a result, OsJAZ1 proteins became more stable, repressing JA responses, thus causing the formation of abnormal spikelet structures. Moreover, we discovered that JA signaling reduced the OsPRMT6a mRNA level in an OsMYC2-dependent manner, thereby establishing a negative feedback loop to balance JA signaling. Furthermore, we found that OsPRMT6a-mediated arginine methylation of OsJAZ1 likely serves as a switch to tune JA signaling to maintain normal spikelet development under harsh environmental conditions such as high temperatures. Thus, our study established a direct molecular link between arginine methylation and the JA signaling pathway.
Authors: Rina Saito, Yuho Nishizato, Tsumugi Kitajima, Misuzu Nakayama, Yousuke Takaoka, Nobuki Kato and Minoru Ueda.
bioRxiv (2024)
Abstract: "(+)-cis-12-oxo-phytodienoic acid (cis-OPDA) is a biosynthetic precursor of the plant hormone (+)-7-iso-jasmonoyl-L-isoleucine (JA-Ile). It functions as an endogenous chemical signal independent of the JA-Ile receptor COI1-JAZ in Arabidopsis thaliana. The bioactive form of cis-OPDA that induces COI1-JAZ-independent gene expression remains unknown. In this study, we hypothesized that the genuine bioactive forms of cis-OPDA are the downstream metabolites, which upregulate the expression of the OPDA marker genes such as ZAT10/ERF5 in a JA-Ile-independent manner. These downstream metabolites function independently of the JA-Ile-COI1-JAZ-MYCs canonical jasmonate signaling module, and its electrophilic nature is essential for its bioactivity."
Authors: Tim Guntelmann, Karl-Josef Dietz and Harald Gröger.
Organic & Biomolecular Chemistry (2024)
Abstract: "Besides its native biological function as plant hormone, cis-(+)-12-oxo-phytodienoic acid (12-OPDA) serves as a metabolite for the cellular formation of (-)-jasmonic acid and also turned out to have an influence on mammalian cells. In order to make this biologically active, but at the same time very expensive natural product 12-OPDA broadly accessible for further biological and medicinal research, we developed an efficient bioprocess based on the utilization of a tailor-made whole-cell catalyst and following the principles of its biosynthesis in nature. After process optimization, the designed three-step one-pot synthesis of 12-OPDA starting from readily accessible α-linolenic acid was able to be conducted at technically relevant substrate loadings in the range of 5-20 g/L. The desired 12-OPDA was obtained with excellent conversion efficiency and by means of a developed efficient downstream-processing, this emulsifying as well as stereochemically labile biosynthetic metabolite 12-OPDA was then obtained with very high chemical purity (>99%) and enantio- and diastereomeric excess (>99% ee, 96% de) as well as neglectable by-product formation (<1%). With respect to a future technical application, we also demonstrated the scalability of the production of the whole cell-biocatalyst in a high-cell-density fermentation process."
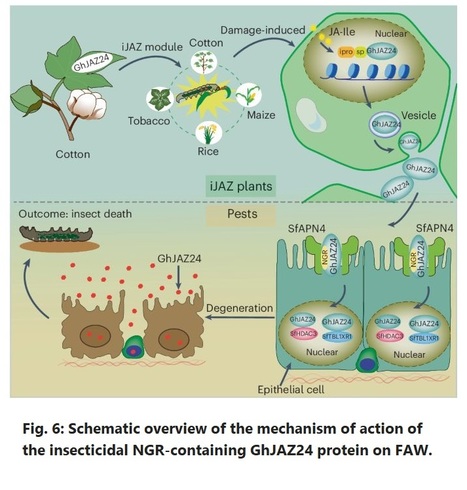
Authors: Huijuan Mo, Huimin Chang, Ge Zhao, Guanjing Hu, Xiumei Luo, Xue Jia, Zhenlu Xu, Guangming Ren, Li Feng, Jonathan F. Wendel, Xiaoya Chen, Maozhi Ren and Fuguang Li.
Nature Plant (2024)
Editor's view: In this study, Mo et al. describe a novel biotechnological strategy to produce crop plants resistant to lepidopteran pests.
Abstract: "The fall armyworm (FAW) poses a significant threat to global crop production. Here we showed that overexpression of jasmonate ZIM-domain (JAZ) protein GhJAZ24 confers resistance to cotton bollworm and FAW, while also causing sterility in transgenic cotton by recruiting TOPLESS and histone deacetylase 6. We identified the NGR motif of GhJAZ24 that recognizes and binds the aminopeptidase N receptor, enabling GhJAZ24 to enter cells and disrupt histone deacetylase 3, leading to cell death. To overcome plant sterility associated with GhJAZ24 overexpression, we developed iJAZ (i, induced), an approach involving damage-induced expression and a switch from intracellular to extracellular localization of GhJAZ24. iJAZ transgenic cotton maintained fertility and showed insecticidal activity against cotton bollworm and FAW. In addition, iJAZ transgenic rice, maize and tobacco plants showed insecticidal activity against their lepidopteran pests, resulting in an iJAZ-based approach for generating alternative insecticidal proteins with distinctive mechanisms of action, thus holding immense potential for future crop engineering."
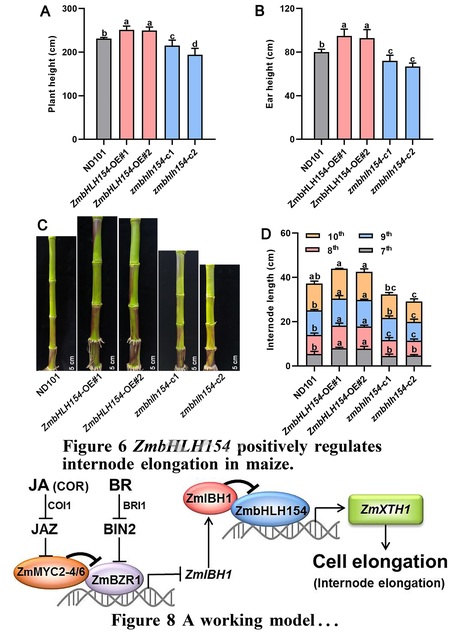
Authors: Xing Wang, Zhaobin Ren, Shipeng Xie, Zhaohu Li, Yuyi Zhou and Liusheng Duan.
Plant Physiology (2024)
One-sentence summary: A jasmonate mimic regulates a basic helix-loop-helix network by attenuating brassinosteroid signaling, which represses expression of a cell wall–related gene and inhibits internode elongation in maize.
Abstract: "Lodging restricts growth, development, and yield formation in maize (Zea mays L.). Shorter internode length is beneficial for lodging tolerance. However, although brassinosteroids (BRs) and jasmonic acid (JA) are known to antagonistically regulate internode growth, the underlying molecular mechanism is still unclear. In this study, application of the JA mimic coronatine (COR) inhibited basal internode elongation at the jointing stage and repressed expression of the cell wall-related gene XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE 1 (ZmXTH1), whose overexpression in maize plants promotes internode elongation. We demonstrated that the basic helix–loop–helix (bHLH) transcription factor ZmbHLH154 binds directly to the ZmXTH1 promoter and induces its expression, whereas the bHLH transcription factor ILI1 BINDING BHLH 1 (ZmIBH1) inhibits this transcriptional activation by forming a heterodimer with ZmbHLH154. Overexpressing ZmbHLH154 led to longer internodes, whereas zmbhlh154 mutants had shorter internodes than the wild type. The core JA-dependent transcription factors ZmMYC2-4 and ZmMYC2-6 interacted with BRASSINAZOLE RESISTANT 1 (ZmBZR1), a key factor in BR signaling, and these interactions eliminated the inhibitory effect of ZmBZR1 on its downstream gene ZmIBH1. Collectively, these results reveal a signaling module in which JA regulates a bHLH network by attenuating BR signaling to inhibit ZmXTH1 expression, thereby regulating cell elongation in maize."
Authors: Lijuan Zhu, Haitao Yu, Xiaoyu Xu and Zhifang Yu.
Postharvest Biology and Technology (2024)
Highlights: • 10 μM MeJA inhibited ethylene biosynthesis and maintained better fruit quality in peach. • JA-biosynthetic pathway enhanced by MeJA was repressed during storage. • MeJA activated the negative feedback of JA-signaling pathway in peach.
Abstract: "Peach undergoes a rapid ripening and senescence, resulting in quality deterioration after harvest. Ethylene is essential for ripening and senescence in climacteric fruit. Although methyl jasmonate (MeJA) has been found to suppress ethylene biosynthesis, the underlying mechanism is unknown. We investigated the regulation of MeJA treatment (10 μM, 24 h) on the quality, ethylene biosynthesis and signaling, and jasmonic acid (JA) metabolism and signaling in ‘Xiahui 8’ peach stored at 20 °C. MeJA treatment maintained better fruit quality and reduced ethylene production (the reduction of peak value reached 24 %) during storage. The activities of 1-aminocyclopropane-1-carboxylate synthase (ACS) and 1-aminocyclopropane-1-carboxylate oxidase (ACO) decreased by 15–28 % and 15–19 % in MeJA treated fruit during storage. Additionally, the expressions of genes involved in ethylene signaling were downregulated in MeJA treated fruit. MeJA treatment enhanced JA biosynthesis through increasing the activities of allene oxide synthase (AOS), allene oxide cyclase (AOC) and JA-amino synthetase (JAR) during early storage. However, the JA-inducible catabolic pathways and self-repression of myelocytomatosis proteins2 (MYC2) were activated in MeJA treated fruit thereafter. This resulted in the decrease of PpMYC2 transcription and JA-Ile accumulation, which reduced by at most 56 % and 73 % when compared with CK, respectively, on day 0 and day 5. These results suggested that negative feedback regulation terminated and repressed the JA signaling, therefore diminishing its enhancement effect on ethylene biosynthesis in peach during storage."
Authors: Grace A. Johnston, Hannah M. Berry, Mikiko Kojima, Hitoshi Sakakibara and Cristiana T. Argueso.
bioRxiv (2024)
Abstract: "Plant immunity activation often results in suppression of plant growth, particularly in the case of constitutive immune activation. We discovered that signaling of the phytohormone cytokinin (CK), known to regulate plant growth through the control of cell division and shoot apical meristem (SAM) activity, can be suppressed by negative crosstalk with the defense phytohormones jasmonic acid (JA), and most evidently, salicylic acid (SA). We show that changing the negative crosstalk of SA on CK signaling in autoimmunity mutants by targeted increase of endogenous CK levels results in plants resistant to pathogens from diverse lifestyles, and relieves suppression of reproductive growth. Moreover, such changes in crosstalk result in a novel reproductive growth phenotype, suggesting a role for defense phytohormones in the SAM, likely through regulation of nitrogen response and cellular redox status. Our data suggest that targeted phytohormone crosstalk engineering can be used to achieve increased reproductive growth and pathogen resistance."
Authors: Wei Wang, Jinyao Ouyang, Yating Li, Changsheng Zhai, Bing He, Huahan Si, Kunsong Chen, Jocelyn K.C. Rose and Wensuo Jia.
Journal of Integrative Plant Biology (2024)
Abstract: "It is generally accepted that jasmonate-ZIM domain (JAZ) repressors act to mediate jasmonate (JA) signaling via CORONATINE-INSENSITIVE1 (COI1)-mediated degradation. Here, we report a cryptic signaling cascade where a JAZ repressor, FvJAZ12, mediates multiple signaling inputs via phosphorylation-modulated subcellular translocation rather than the COI1-mediated degradation mechanism in strawberry (Fragaria vesca). FvJAZ12 acts to regulate flavor metabolism and defense response, and was found to be the target of FvMPK6, a mitogen-activated protein kinase that is capable of responding to multiple signal stimuli. FvMPK6 phosphorylates FvJAZ12 at the amino acid residues S179 and T183 adjacent to the PY residues, thereby attenuating its nuclear accumulation and relieving its repression for FvMYC2, which acts to control the expression of lipoxygenase 3 (FvLOX3), an important gene involved in JA biosynthesis and a diverse array of cellular metabolisms. Our data reveal a previously unreported mechanism for JA signaling and decipher a signaling cascade that links multiple signaling inputs with fruit trait development."
Authors: Yuqing He, Yao Zhao, Jitao Hu, Lanlan Wang, Linying Li, Xueying Zhang, Zhongjing Zhou, Lili Chen, Hua Wang, Jiaoyu Wang and Gaojie Hong.
Molecular Plant (2024)
Abstract: "The growth-promoting hormones brassinosteroids (BRs) and their key signaling component BZR1 play a vital role in balancing normal growth and defense reactions. Here, we discovered that BRs and OsBZR1 upregulated sakuranetin accumulation and conferred basal defense against Magnaporthe oryzae infection under normal conditions. Resource shortages, including phosphate (Pi) deficiency, potentially disrupt this growth–defense balance. OsSPX1 and OsSPX2 have been reported to sense Pi concentration and interact with the Pi signal mediator OsPHR2, thus regulating Pi starvation responses. In this study, we discovered that OsSPX1/2 interacts with OsBZR1 in both Pi-sufficient and Pi-deficient conditions, inhibiting BR-responsive genes. When Pi is sufficient, OsSPX1/2 is captured by OsPHR2, enabling most of OsBZR1 to promote plant growth and maintain basal resistance. In response to Pi starvation, more OsSPX1/2 is released from OsPHR2 to inhibit OsBZR1 activity, resulting in slower growth. Collectively, our study reveals that the OsBZR1–SPX1/2 module balances the plant growth–immunity trade-off in response to Pi availability."
Authors: Yajun Gou, Yueqin Heng, Wenyan Ding, Canhong Xu, Qiushuang Tan, Yajing Li, Yudong Fang, Xiaoqing Li, Degui Zhou, Xinyu Zhu, Mingyue Zhang, Rongjian Ye, Haiyang Wang and Rongxin Shen.
Nature Communications (2024)
Editor's view: Florets of indica rice open earlier than japonica rice, hindering utilization of the cross subspecies heterosis. Here, the authors show that an OsMYB8-OsJAR1 module regulates diurnal floret opening time divergences between the two subspecies.
Abstract: "The inter-subspecific indica-japonica hybrid rice confer potential higher yield than the widely used indica-indica intra-subspecific hybrid rice. Nevertheless, the utilization of this strong heterosis is currently hindered by asynchronous diurnal floret opening time (DFOT) of indica and japonica parental lines. Here, we identify OsMYB8 as a key regulator of rice DFOT. OsMYB8 induces the transcription of JA-Ile synthetase OsJAR1, thereby regulating the expression of genes related to cell osmolality and cell wall remodeling in lodicules to promote floret opening. Natural variations of OsMYB8 promoter contribute to its differential expression, thus differential transcription of OsJAR1 and accumulation of JA-Ile in lodicules of indica and japonica subspecies. Furthermore, introgression of the indica haplotype of OsMYB8 into japonica effectively promotes DFOT in japonica. Our findings reveal an OsMYB8-OsJAR1 module that regulates differential DFOT in indica and japonica, and provide a strategy for breeding early DFOT japonica to facilitate breeding of indica-japonica hybrids."
Authors: Mumei Wang, Xiaopei Zhu, Zhen Huang, Minghao Chen, Peng Xu, Shitang Liao, Yongzhen Zhao, Yannan Gao, Jiahui He, Yutong Luo, Huixuan Chen, Xiaoying Wei, Shuai Nie, Jingfang Dong, Liya Zhu, Chuxiong Zhuang, Junliang Zhao, Zhenlan Liu and Hai Zhou.
Plant Biotechnology Journal (2024)
Abstract: "Inter-subspecific indica–japonica hybrid rice (Oryza sativa) has the potential for increased yields over traditional indica intra-subspecies hybrid rice, but limited yield of F1 hybrid seed production (FHSP) hinders the development of indica–japonica hybrid rice breeding. Diurnal flower-opening time (DFOT) divergence between indica and japonica rice has been a major contributing factor to this issue, but few DFOT genes have been cloned. Here, we found that manipulating the expression of jasmonate (JA) pathway genes can effectively modulate DFOT to improve the yield of FHSP in rice. Treating japonica cultivar Zhonghua 11 (ZH11) with methyl jasmonate (MeJA) substantially advanced DFOT. Furthermore, overexpressing the JA biosynthesis gene OPDA REDUCTASE 7 (OsOPR7) and knocking out the JA inactivation gene CHILLING TOLERANCE 1 (OsHAN1) in ZH11 advanced DFOT by 1- and 2-h respectively; and knockout of the JA signal suppressor genes JASMONATE ZIM-DOMAIN PROTEIN 7 (OsJAZ7) and OsJAZ9 resulted in 50-min and 1.5-h earlier DFOT respectively. The yields of FHSP using japonica male-sterile lines GAZS with manipulated JA pathway genes were significantly higher than that of GAZS wildtype. Transcriptome analysis, cytological observations, measurements of elastic modulus and determination of cell wall components indicated that the JA pathway could affect the loosening of the lodicule cell walls by regulating their composition through controlling sugar metabolism, which in turn influences DFOT. This research has vital implications for breeding japonica rice cultivars with early DFOT to facilitate indica–japonica hybrid rice breeding."
|


 Your new post is loading...
Your new post is loading...