Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
From
doi
Authors: Juncai Deng, Xiangqing Huang, Jianhua Chen, Bartel Vanholme, Jinya Guo, Yuanyuan He, Wenting Qin, Jing Zhang, Wenyu Yang and Jiang Liu.
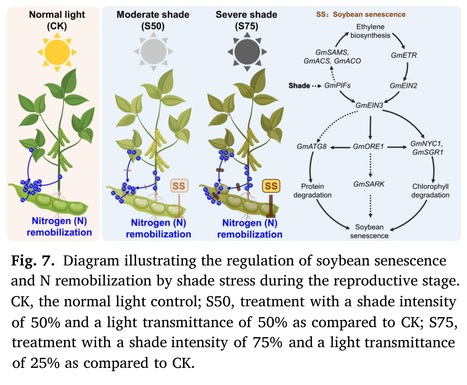
Plant Physiology and Biochemistry (2024) Highlights: • Shade stress leads to premature senescence in soybean plants. • Ethylene biosynthesis and signal transduction are induced to regulate soybean plant senescence. • Shade stress retains nitrogen in soybean vegetative organs and impedes the remobilization of nitrogen from these organs. • These negative impacts can be mitigated by reducing the intensity of shade stress through field layout optimization. Abstract: "In gramineae-soybean intercropping systems, shade stress caused by taller plants impacts soybean growth specifically during the reproductive stage. However, the effects of shade stress on soybean senescence remain largely unexplored. In this research, we applied artificial shade treatments with intensities of 75% (S75) and 50% (S50) to soybean plants at the onset of flowering to simulate the shade stress experienced by soybeans in the traditional and optimized maize-soybean intercropping systems, respectively. Compared to the normal light control, both shade treatments led to a rapid decline in the dry matter content of soybean vegetative organs and accelerated their abscission. Moreover, shade treatments triggered the degradation of chlorophyll and soluble proteins in leaves and increased the expression of genes associated with leaf senescence. Metabolic profiling further revealed that ethylene biosynthesis and signal transduction were induced by shade treatment. In addition, the examination of nitrogen content demonstrated that shade treatments impeded the remobilization of nitrogen in vegetative tissues, consequently reducing the seed nitrogen harvest. It's worth noting that these negative effects were less pronounced under the S50 treatment compared to the S75 treatment. Taken together, this research demonstrates that shade stress during the reproductive stage accelerates soybean senescence and impedes nitrogen remobilization, while optimizing the field layout to improve soybean growth light conditions could mitigate these challenges in the maize-soybean intercropping system."
From
doi
Authors: Jihong Li and Yuan Song.
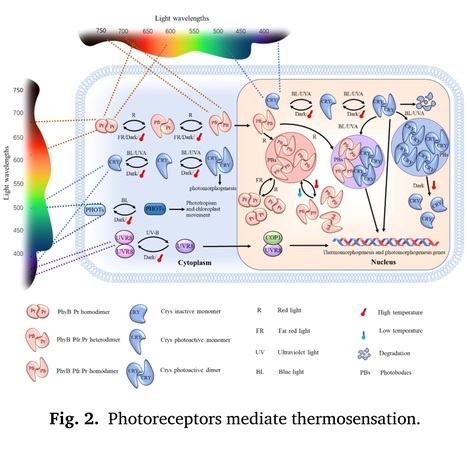
Plant Science (2024) Highlights: • The research of plant thermosensor is obviously lagging behind and hence has become an urgent problem to be resolved. • We summarized the recognized and potential plant thermosensors,to describe the multi–level thermal input system in plants. • We reviewed more recent thermosensing mechanisms to facilitate further understanding and studies. Abstract: "Plants dynamically regulate their genes expression and physiological outputs to adapt to changing temperatures. The underlying molecular mechanisms have been extensively studied in diverse plants and in multiple dimensions. However, the question of exactly how temperature is detected at molecular level to transform the physical information into recognizable intracellular signals remains continues to be one of the undetermined occurrences in plant science. Recent studies have provided the physical and biochemical mechanistic breakthrough of how temperature changes can influence molecular thermodynamically stability, thus changing molecular structures, activities, interaction and signaling transduction. In this review, we focus on the thermosensing mechanisms of recognized and potential plant thermosensors, to describe the multi–level thermal input system in plants. We also consider the attributes of a thermosensor on the basis of thermal-triggered changes in function, structure, and physical parameters. This study thus provides a reference for discovering more plant thermosensors and elucidating plant thermal adaptive mechanisms."
Julio Retamales's insight:
Relevant review!
Authors: Junling Huai, Nan Gao, Yuanyuan Yao, Yanxin Du, Qiang Guo and Rongcheng Lin. Plant Physiology (2024) One-sentence summary: A jasmonic acid signaling component regulates Arabidopsis photo- and thermo-morphogenesis by repressing PHYTOCHROME-INTERACTING FACTOR4. Abstract: "Light and temperature are two major environmental factors that affect growth and development of plants during their life cycle. Plants have evolved complex mechanisms to adapt to varying external environments. Here, we show that JASMONATE ZIM-domain protein 3 (JAZ3), a jasmonic acid signaling component, acts as a factor to integrate light and temperature in regulating seedling morphogenesis. JAZ3 overexpression transgenic lines display short hypocotyls under red, far-red, and blue light and warm temperature (28 °C) conditions compared to the wild type in Arabidopsis (Arabidopsis thaliana). We show that JAZ3 interacts with the transcription factor PHYTOCHROME-INTERACTING FACTOR4 (PIF4). Interestingly, JAZ3 spontaneously undergoes liquid-liquid phase separation (LLPS) in vitro and in vivo and promotes LLPS formation of PIF4. Moreover, transcriptomic analyses indicate that JAZ3 regulates the expression of genes involved in many biological processes, such as response to auxin, auxin-activated signaling pathway, regulation of growth, and response to red light. Finally, JAZ3 inhibits the transcriptional activation activity and binding ability of PIF4. Collectively, our study reveals a function and molecular mechanism of JAZ3 in regulating plant growth in response to environmental light and temperature."
Julio Retamales's insight:
Good paper!
From
www
Authors: Linge Li, Jesse Wonder, Ticho Helming, Gijs van Asselt, Chrysoula K. Pantazopoulou, Yorrit van de Kaa, Wouter Kohlen, Ronald Pierik and Kaisa Kajala. bioRxiv (2023) Abstract: "In this study, we explore the dynamic interplay between the plant hormones gibberellins (GA), brassinosteroids (BR), and Indole-3-Acetic Acid (IAA) in their collective impact on plant shade avoidance elongation under varying light conditions. We focus particularly on low Red : Far-red (R:FR) light conditions achieved by supplementing the background light with FR. Our research delves into how these hormones individually and synergistically influence stem elongation in tomato plants. Through meticulous experimental modulations of GA, IAA, and BR, we demonstrate that GA and BR are sufficient but also necessary for inducing stem elongation under low R:FR light conditions. Intriguingly, while IAA alone shows limited effects, its combination with GA yields significant elongation, suggesting a nuanced hormonal balance. Furthermore, we unveil the complex interplay of these hormones under light with low R:FR, where the suppression of one hormone's effect can be compensated by the others. This study provides insights into the hormonal mechanisms governing plant adaptation to light, highlighting the intricate and adaptable nature of plant growth responses. Our findings have far-reaching implications for agricultural practices, offering potential strategies for optimizing plant growth and productivity in various lighting environments."
From
doi
Authors: Fan Xu, Jia Chen, Yingbin Li, Shilin Ouyang, Mengting Yu, Yirong Wang, Xianming Fang, Kai He and Feng Yu.
Developmental Cell (2024) Editor's view: Xu and Chen et al. report that the receptor kinase FERONIA interacts with, phosphorylates, and stabilizes the transcription factor PIF3 to regulate PIEZO expression in Arabidopsis root cap, which drives root penetration into soil. Highlights: • FER participates in Arabidopsis and soybean roots penetration into soil • scRNA-seq profiling of fer-4 root identified PIF3 as a FER-regulated target • FER interacts with, phosphorylates, and stabilizes transcription factor PIF3 • FER-PIF3 module regulates PIEZO expression in Arabidopsis root cap Abstract: "The cotyledons of etiolated seedlings from terrestrial flowering plants must emerge from the soil surface, while roots must penetrate the soil to ensure plant survival. We show here that the soil emergence-related transcription factor PHYTOCHROME-INTERACTING FACTOR 3 (PIF3) controls root penetration via transducing external signals perceived by the receptor kinase FERONIA (FER) in Arabidopsis thaliana. The loss of FER function in Arabidopsis and soybean (Glycine max) mutants resulted in a severe defect in root penetration into agar medium or hard soil. Single-cell RNA sequencing (scRNA-seq) profiling of Arabidopsis roots identified a distinct cell clustering pattern, especially for root cap cells, and identified PIF3 as a FER-regulated transcription factor. Biochemical, imaging, and genetic experiments confirmed that PIF3 is required for root penetration into soil. Moreover, FER interacted with and stabilized PIF3 to modulate the expression of mechanosensitive ion channel PIEZO and the sloughing of outer root cap cells."
Julio Retamales's insight:
Very important paper!
Text of the Figure 7 above: "Figure 7. A working model for the integrated regulation of the emergence of cotyledons from the soil and root penetration into the soil by PIF3 For cotyledon emergence, PIF3 transduces light signals to control mechanical stress caused by soil covering. For root penetration, PIF3 transfers the soil constraints perceived by FER to the transcriptional machinery to mediate root cap cell sloughing and induce the expression of the mechanosensitive ion channel gene PIEZO in the root cap and possibly other unknown factors. In this pathway, FER phosphorylates and stabilizes PIF3, resulting in its higher abundance in the root cap."
Authors: Zhaoheng Lin, Pan Zhu, Liyang Gao, Xuanyi Chen, Meijing Li, Yuhe Wang, Junxian He, Ying Miao and Rui Miao.
Plant and Cell Physiology (2024) Abstract: "The polyhydroxylated steroid phytohormone brassinosteroids (BRs) control many aspects of plant growth, development and responses to environmental changes. Plasma membrane (PM) H+-ATPase, the well-known PM proton pump, is a central regulator in plant physiology, which mediates not only plant growth and development, but also adaptation to stresses. Recent studies highlight that PM H+-ATPase is at least partly regulated via the BR signaling. Firstly, the BR cell surface receptor BRASSINOSTEROID-INSENSITIVE 1 (BRI1) and multiple key components of BR signaling directly or indirectly influence PM H+-ATPase activity. Secondly, the SMALL AUXIN UP RNA (SAUR) gene family physically interacts with BRI1 to enhance organ development of Arabidopsis by activating PM H+-ATPase. Thirdly, RNA-sequencing (RNA-seq) assays showed that the expression of some SAUR genes is upregulated under the light or sucrose conditions, which is related to the phosphorylation state of the penultimate residue of PM H+-ATPase in a time-course manner. In this review, we describe the structural and functional features of PM H+-ATPase, and summarize recent progress toward understanding the regulatory mechanism of PM H+-ATPase by BRs, and briefly introduce how PM H+-ATPase activity is modulated by its own biterminal regions and the post-translational modifications."
From
doi
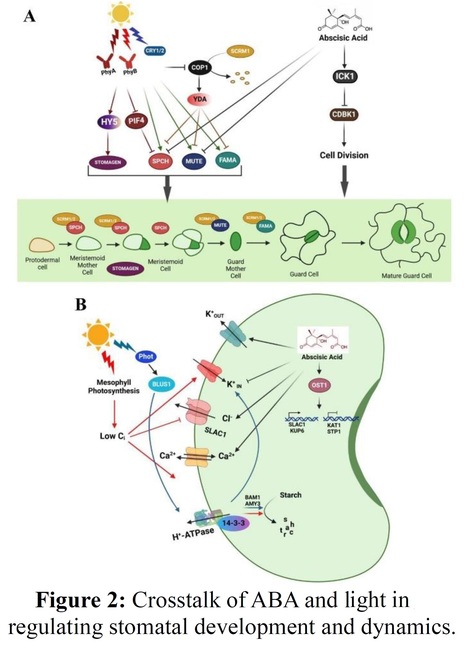
Authors: Jingbo Zhang, Xuexue Chen, Yajing Song and Zhizhong Gong. Journal of Integrative Plant Biology (2024) One-sentence summary: This review summarizes current knowledge on the molecular mechanisms of stomatal movements mediated by abscisic acid, light, CO2, reactive oxygen species, pathogens, temperature, and other phytohormones, which helps in designing smart crops with higher resilience. Abstract: "Global climate change-caused drought stress, high temperatures and other extreme weather profoundly impact plant growth and development, restricting sustainable crop production. To cope with various environmental stimuli, plants can optimize the opening and closing of stomata to balance CO2 uptake for photosynthesis and water loss from leaves. Guard cells perceive and integrate various signals to adjust stomatal pores through turgor pressure regulation. Molecular mechanisms and signaling networks underlying the stomatal movements in response to environmental stresses have been extensively studied and elucidated. This review focuses on the molecular mechanisms of stomatal movements mediated by abscisic acid, light, CO2, reactive oxygen species, pathogens, temperature, and other phytohormones. We discussed the significance of elucidating the integrative mechanisms that regulate stomatal movements in helping design smart crops with enhanced water use efficiency and resilience in a climate-changing world."
Julio Retamales's insight:
Thorough and updated review!
From
doi
Authors: Guy Golan, Jacob Weiner, Yusheng Zhao and Thorsten Schnurbusch.
New Phytologist (2024) Abstract: "How plants distribute biomass among organs influences resource acquisition, reproduction and plant–plant interactions, and is essential in understanding plant ecology, evolution, and yield production in agriculture. However, the genetic mechanisms regulating allocation responses to the environment are largely unknown. We studied recombinant lines of wheat (Triticum spp.) grown as single plants under sunlight and simulated canopy shade to investigate genotype-by-environment interactions in biomass allocation to the leaves, stems, spikes, and grains. Size-corrected mass fractions and allometric slopes were employed to dissect allocation responses to light limitation and plant size. Size adjustments revealed light-responsive alleles associated with adaptation to the crop environment. Combined with an allometric approach, we demonstrated that polymorphism in the DELLA protein is associated with the response to shade and size. While a gibberellin-sensitive allelic effect on stem allocation was amplified when plants were shaded, size-dependent effects of this allele drive allocation to reproduction, suggesting that the ontogenetic trajectory of the plant affects the consequences of shade responses for allocation. Our approach provides a basis for exploring the genetic determinants underlying investment strategies in the face of different resource constraints and will be useful in predicting social behaviours of individuals in a crop community.
Julio Retamales's insight:
Relevant findings!
From
doi
Authors: Jiaojiao Li, Jian Zeng, Zhaoxia Tian and Zhong Zhao.
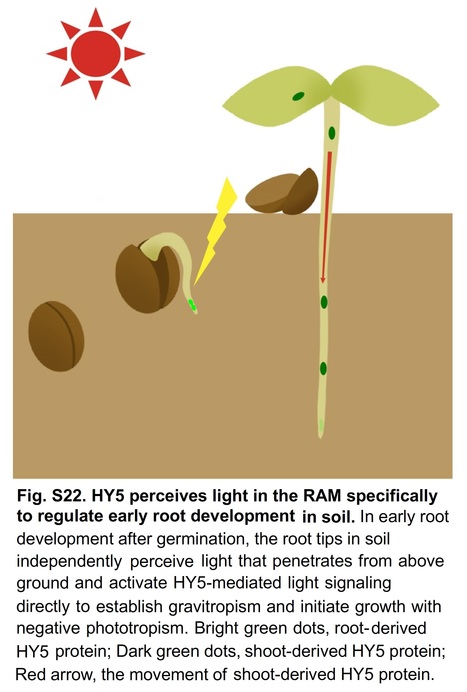
PNAS (2024) Significance: Despite growth beneath the soil, the development of roots is tightly controlled by light signaling, the response of which is thought to depend on signal transmission from the shoot. However, whether roots are capable of specifically perceiving light remains unclear. This research shows that the root apical meristem (RAM) perceives light independently from aboveground organs to direct early root development by the light-regulated transcription factor ELONGATED HYPOCOTYL5 (HY5). Root-derived HY5 activates peroxidase expression directly or indirectly by repressing the peroxidase inhibitor to eliminate H2O2 in the RAM to promote root development. Our data suggest that HY5 effects photoreception in the RAM and integrates ROS signaling to direct early root development. Abstract: "Root development is tightly controlled by light, and the response is thought to depend on signal transmission from the shoot. Here, we show that the root apical meristem perceives light independently from aboveground organs to activate the light-regulated transcription factor ELONGATED HYPOCOTYL5 (HY5). The ROS balance between H2O2 and superoxide anion in the root is disturbed under darkness with increased H2O2. We demonstrate that root-derived HY5 directly activates PER6 expression to eliminate H2O2. Moreover, HY5 directly represses UPBEAT1, a known inhibitor of peroxidases, to release the expression of PERs, partially contributing to the light control of ROS balance in the root. Our results reveal an unexpected ability in roots with specific photoreception and provide a mechanistic framework for the HY5-mediated interaction between light and ROS signaling in early root development."
Julio Retamales's insight:
Major finding: light is perceived directly in the root apical meristem...
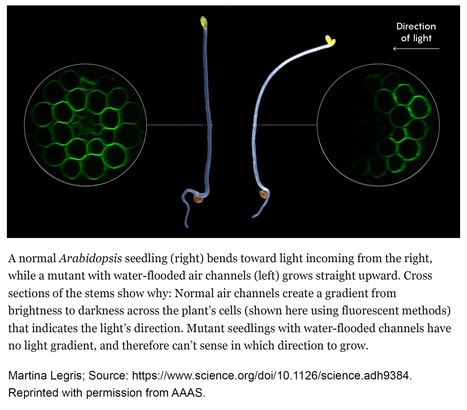
Author: Asher Elbein Quanta Magazine (2024). Subheading: A mutant seedling revealed how plant tissues scatter incoming light, allowing plants to sense its direction and move toward it. Excerpts: "But in the absence of obvious physical sensing organs like lenses, how do plants work out the precise direction from which light is coming? Now, a team of European researchers has hit upon an answer. In a recent paper published in Science, they report that a roadside weed — Arabidopsis, a favorite of plant geneticists — uses the air spaces between its cells to scatter light, modifying the path of light passing through its tissues. In this way, the air channels create a light gradient that helps seedlings accurately determine where light is coming from. By taking advantage of air channels to scatter light, plants sidestep the need for discrete organs like eyes in favor of a neater trick: the ability in effect to “see” with their whole bodies." "The researchers deduced that the plant orients itself to light through a mechanism based on the phenomenon of refraction — the tendency of light to change direction as it passes through different media. Because of refraction, Legris explained, light passing through a normal Arabidopsis will scatter under the surface of the stem: Every time it moves through a plant cell, which is mostly water, and then through an air channel, it changes direction. Since some of the light is redirected in the process, the air channels establish a steep light gradient across different cells, which the plant can use to assess the light’s direction and then grow toward it."
Julio Retamales's insight:
An excellent commentary (and much more) on the fascinating paper by Nawkar at al. ("Air channels create a directional light signal to regulate hypocotyl phototropism"), which was already posted here and is to be found at:
Authors: Yongfeng Gao, Zihao Chen, Qian Feng, Tao Long, Jihua Ding, Peng Shu, Heng Deng, Peizhi Yu, Wenrong Tan, Siqin Liu, Lucas Gutierrez Rodriguez, Lijun Wang, Víctor Resco de Dios and Yinan Yao.
The Plant Cell (2024) Abstract: "Photoperiod is a crucial environmental cue for phenological responses, including growth cessation and winter dormancy in perennial woody plants. Two regulatory modules within the photoperiod pathway explain bud dormancy induction in poplar (Populus spp.): the circadian oscillator LATE ELONGATED HYPOCOTYL 2 (LHY2) and GIGANTEA-like genes (GIs) both regulate the key target for winter dormancy induction FLOWERING LOCUS T2 (FT2). However, modification of LHY2 and GIs cannot completely prevent growth cessation and bud set under short-day conditions, indicating that additional regulatory modules are likely involved. We identified PtoHY5a, an orthologs of the photomorphogenesis regulatory factor ELONGATED HYPOCOTYL 5 (HY5) in poplar (Populus tomentosa), that directly activates PtoFT2 expression and represses the circadian oscillation of LHY2, indirectly activating PtoFT2 expression. Thus, PtoHY5a suppresses short day-induced growth cessation and bud set. Accordingly, PtoHY5a knockout facilitates dormancy induction. PtoHY5a also inhibits bud-break in poplar by controlling gibberellic acid (GA) levels in apical buds. Additionally, PtoHY5a regulates the photoperiodic control of seasonal growth downstream of phytochrome PHYB2. Thus, PtoHY5a modulates seasonal growth in poplar by regulating the PtoPHYB2–PtoHY5a–PtoFT2 module to determine the onset of winter dormancy, and by fine-tuning GA levels to control bud-break."
Authors: Esther Cañibano, Daniela Soto-Gomez, Juan Carlos Oliveros, Clara Bourbousse and Sandra Fonseca. bioRxiv (2024) Abstract: "Driven by cell elongation, hypocotyl growth is tightly controlled by light and responds to external stimuli and endogenous hormonal pathways. Hypocotyls are known to be responsive to the stress signalling hormone abscisic acid (ABA) which effectively inhibits cell elongation, but how this regulation is connected to light responses and other endogenous hormonal pathways has been a subject of limited studies. Here, we show that whereas hypocotyl elongation is sensitive to ABA in light-grown seedlings, the hypocotyl of dark-grown etiolated seedlings is ABA-insensitive. In the dark, hypocotyl sensitivity to ABA is restored in the constitutive photomorphogenic pifq and cop1-4 mutants, suggesting that an active light signalling pathway is necessary for hypocotyl responsiveness to ABA. However, etiolated hypocotyls retain ABA responsiveness, as could be detected by the induction of ABI1 and RD29B transcripts in response to exogenous ABA, suggesting that inhibition of hypocotyl elongation mediated by ABA does not follows the canonical ABA signalling dependent on transcription. Here, using RNA-seq analysis we identified a number of ABA differentially expressed genes (DEGs) that correlate with ABA inhibition of hypocotyl elongation, specifically in dark-grown pifq or light-grown WT plants, and whose expression remains unchanged by ABA treatment in dark-grown WT plants. Among these DEGs we identified a number of genes playing a role in cell elongation directly at the level of the plasma membrane, as SAURs, ion transporters, auxin flux regulators, channels, and cell wall modification enzymes. The use of the auxin transport inhibitor, NPA, revealed that in the light auxin transport impairment renders hypocotyls insensitive to ABA in WT and pifq plants. Thus, in the light, hypocotyl responsiveness to ABA is dependent on auxin transport and independent of PIFs. In the dark, PIFs render hypocotyls insensitive to ABA, perhaps by regulating the expression of a number of ABA DEGs, a mechanism that could allow plants to prioritize the elongation towards light, avoiding to slow-down soil emergence that could be induced by ABA signalling in case of sudden reduction of soil moisture."
Julio Retamales's insight:
Relevant findings!
|
Authors: Hiroshi Takagi, Nayoung Lee, Andrew K. Hempton, Savita Purushwani, Michitaka Notaguchi, Kota Yamauchi, Kazumasa Shirai, Yaichi Kawakatsu, Susumu Uehara, William G. Albers, Benjamin L. R. Downing, Shogo Ito, Takamasa Suzuki, Takakazu Matsuura, Izumi C. Mori, Nobutaka Mitsuda, Daisuke Kurihara, Tomonao Matsushita, Young Hun Song, Yoshikatsu Sato, Mika Nomoto, Yasuomi Tada, Kousuke Hanada, Josh T. Cuperus, Christine Queitsch and Takato Imaizumi. bioRxiv (2024) Abstract: "Seasonal changes in spring induce flowering by expressing the florigen, FLOWERING LOCUS T (FT), in Arabidopsis. FT is expressed in unique phloem companion cells with unknown characteristics. The question of which genes are co-expressed with FT and whether they have roles in flowering remains elusive. Through tissue-specific translatome analysis, we discovered that under long-day conditions with the natural sunlight red/far-red ratio, the FT-producing cells express a gene encoding FPF1-LIKE PROTEIN 1 (FLP1). The master FT regulator, CONSTANS (CO), controls FLP1 expression, suggesting FLP1's involvement in the photoperiod pathway. FLP1 promotes early flowering independently of FT, is active in the shoot apical meristem, and induces the expression of SEPALLATA 3 (SEP3), a key E-class homeotic gene. Unlike FT, FLP1 facilitates inflorescence stem elongation. Our cumulative evidence indicates that FLP1 may act as a mobile signal. Thus, FLP1 orchestrates floral initiation together with FT and promotes inflorescence stem elongation during reproductive transitions."
Julio Retamales's insight:
Relevant finding!
Text of figure (letter O) shown above: "Figure 7 FLP1 may be a vascular-mobile protein that may function in the tissues distantly from leaves...... (O) A model of coordination of flowering and inflorescence stem growth by FLP1 and FT. CO regulates the expression of both FLP1 and FT in photoperiod- and far-red light-dependent manners. FT is synthesized in specific leaf phloem companion cells in response to environmental stimuli and moved to the SAM to induce flowering by directly upregulating LFY and AP1. FLP1 is also synthesized in the same cells where FT is synthesized. It can move through the phloem, and possibly the movement of FLP1 may be important for promoting floral transition through SEP3 induction. FLP1 also participates in the inflorescence stem elongation."
Authors: Kalyan Mahapatra, Shubhi Dwivedi, Arpan Mukherjee, Ajar Anupam Pradhan, Kavuri Venkateswara Rao, Deeksha Singh, Lavanya Bhagavatula and Sourav Datta. Journal of Experimental Botany (2024) Abstract: "The exogenous light cues and the phytohormone Abscisic acid (ABA) regulate several aspects of plant growth and development. In recent years, the role of the crosstalk between the light and ABA signaling pathways in regulating different physiological processes has become increasingly evident. This includes the regulation of germination and early seedling development, control of stomatal development and conductance, growth and development of roots, buds, branches, and regulation of flowering. Light and ABA signaling cascades have various convergence points at both DNA and protein levels. The molecular crosstalk involves several light signaling factors like HY5, COP1, PIFs and BBXs that integrate with ABA signaling components like the PYL receptors and ABI5. Especially, ABI5 and PIF4 promoters serve as key “hotspots” for the integration of these two pathways. Plants acquired both light and ABA signaling pathways before they colonized land almost 500 million years ago. In this review, we discuss the recent advances in the interplay of light and ABA signaling regulating plant development and provide an overview of the evolution of these two pathways."
Author: Kumari Billakurthi. Plant Physiology (2024) Excerpts: "In this issue of Plant Physiology, Huai and co-workers (Huai et al., 2024) investigated a molecular mechanism by which JA signaling integrates light and temperature responses in shaping seedling morphogenesis in Arabidopsis. Among 13 JAZ genes in Arabidopsis, the authors found that overexpression of JAZ3 (35S:JAZ3-GFP) resulted in notable phenotypic changes such as shorter hypocotyls and cotyledon unfolding under various light conditions (continuous red, far-red, blue, and low-intensity white light conditions). However, phenotypes of JAZ3-GFP and jaz3 mutant lines did not differ from wild type in the dark. Furthermore, the study revealed that overexpression of JAZ3 inhibited warm temperature-mediated hypocotyl elongation at 28°C, underscoring its role in thermomorphogenesis as well. This dual regulation of seedling morphogenesis by JAZ3 suggests its significance in coordinating responses to light and temperature cues." "In summary, this study uncovers the crucial role of JAZ3 in integrating light and temperature signaling pathways in Arabidopsis seedling morphogenesis. By interacting with PIF4 and promoting its phase separation, JAZ3 acts as a critical regulator, modulating PIF4-mediated responses, such as hypocotyl elongation and cotyledon expansion (Figure 1)."
Julio Retamales's insight:
Commentary on the relevant article by Huai et al. (" JASMONATE ZIM-DOMAIN PROTEIN 3 regulates photo- and thermo-morphogenesis through inhibiting PIF4 in Arabidopsis."), which is also posted here.
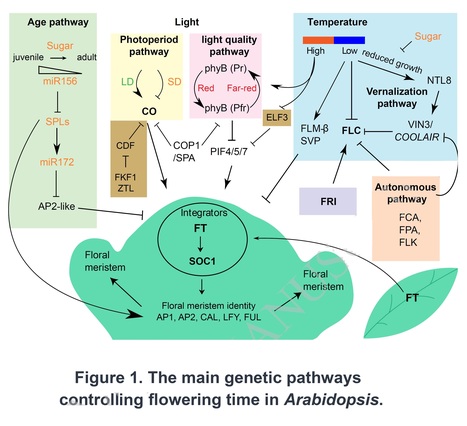
Authors: Robert Maple, Pan Zhu, Jo Hepworth, Jia-Wei Wang and Caroline Dean.
Plant Physiology (2024) Abstract: "Plant species have evolved different requirements for environmental/endogenous cues to induce flowering. Originally, these varying requirements were thought to reflect the action of different molecular mechanisms. Thinking changed when genetic and molecular analysis in Arabidopsis thaliana revealed that a network of environmental and endogenous signalling input pathways converge to regulate a common set of ‘floral pathway integrators’. Variation in the predominance of the different input pathways within a network can generate the diversity of requirements observed in different species. Many genes identified by flowering time mutants were found to encode general developmental and gene regulators, with their targets having a specific flowering function. Studies of natural variation in flowering were more successful at identifying genes acting as nodes in the network central to adaptation and domestication. Attention has now turned to mechanistic dissection of flowering time gene function and how that has changed during adaptation. This will inform breeding strategies for climate-proof crops and help define which genes act as critical flowering nodes in many other species."
Julio Retamales's insight:
Outstanding review!
Text of the figure above: "Figure 1. The main genetic pathways controlling flowering time in Arabidopsis. Colored boxes highlight different pathways; FRI (purple) and clock components (brown), key integration nodes (FLC, CO, FT, and SOC1), and those with extensive natural variation (FRIGIDA, FLC, and FT) are in bold. Arrows indicate positive and bars represent negative regulatory relationships. Genetic pathways converge on FT, encoding a transmissible signaling molecule transported from the leaves to the SAM. The floral pathway integrators (in a circle) and floral meristem identity genes are shown in the green schematic meristem. The influence of sugar on some pathways is indicated. Different pathways are interconnected, e.g., photoperiod and light quality and temperature pathways by COP1/SPA, and circadian and high temperature pathways by ELF3."
From
doi
Authors: llohim Bello-Bello and Luis Herrera-Estrella.
Developmental Cell (2024) Summary: "Penetration capacity is a central root phenotype for enhancing rooting into compacted soils and improving abiotic stress tolerance in crop plants, but the molecular mechanisms are unclear. In this issue of Developmental Cell, Xu et al. demonstrate the vital role of the FER-PIF3-PIEZO circuitry in controlling primary root penetrability into compacted substrates."
Julio Retamales's insight:
Extended commentary on the relevant article by Xu et al. ("The soil emergence-related transcription factor PIF3 controls root penetration by interacting with the receptor kinase FER"), which is also posted here. Text of the figure above: "Figure 1. Model of the molecular mechanisms modulating the inhibition of root elongation and penetration in compacted soil In rice, ethylene activates a EIN3/EIL1-dependent signaling pathway that directs auxin (via YUC8) and ABA biosynthesis (via MHZ5, CRTISO, ZEP, etc.) in roots. Auxin acts as an inhibitor of epidermal cell elongation and, ABA stimulates the expansion of cortical cells; both processes contribute to the inhibition of root elongation in compacted soils. In Arabidopsis, FER phosphorylates and stabilizes PIF3. Then PIF3 regulates the expression of root cap-specific genes, including PIEZO1 and genes associated with sloughing of root cap cells, which help roots to penetrate the compacted soil. EIN3/EIL1 is hypothesized to connect these molecular mechanisms and interfere with both the root responses to compaction and root soil penetration."
From
doi
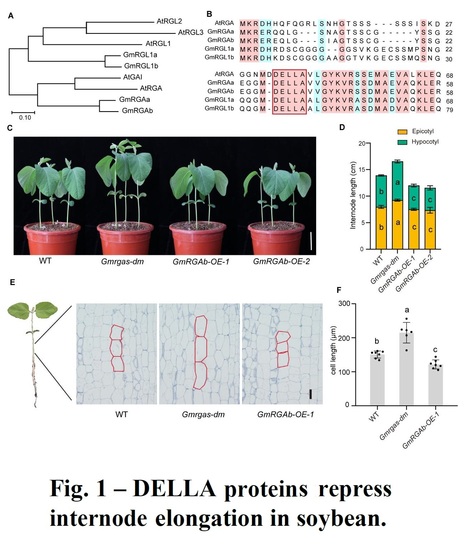
Authors: Zhuang Li, Qichao Tu, Xiangguang Lyu, Qican Cheng, Ronghuan Ji, Chao Qin, Jun Liu, Bin Liu, Hongyu Li and Tao Zhao.
The Crop Journal (2024) Abstract: "Plant height influences plant architecture, lodging resistance, and yield performance. It is modulated by gibberellic acid (GA) metabolism and signaling. DELLA proteins, acting as central repressors of GA signaling, integrate various environmental and hormonal signals to regulate plant growth and development in Arabidopsis. We examined the role of two DELLA proteins, GmRGAa and GmRGAb, in soybean plant height control. Knockout of these proteins led to longer internodes and increased plant height, primarily by increasing cell elongation. GmRGAs functioned under different light conditions, including red, blue, and far-red light, to repress plant height. Interaction studies revealed that GmRGAs interacted with the blue light receptor GmCRY1b. Consistent with this, GmCRY1b partially regulated plant height via GmRGAs. Additionally, DELLA proteins were found to stabilize the protein GmSTF1/2, a key positive regulator of photomorphogenesis. This stabilization led to increased transcription of GmGA2ox-7b and subsequent reduction in plant height. This study enhances our understanding of DELLA-mediated plant height control, offering Gmrgaab mutants for soybean structure and yield optimization.
From
doi
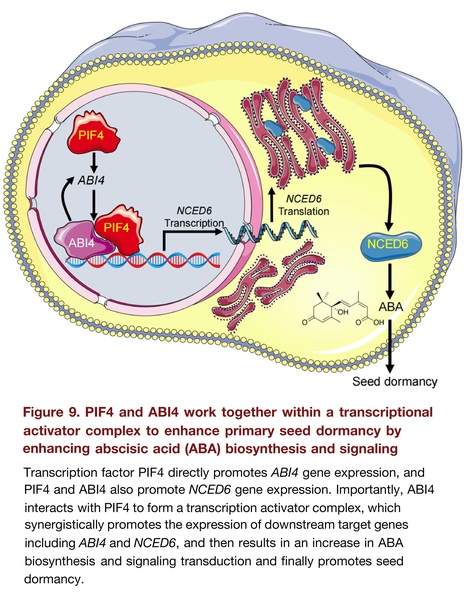
Authors: Xiaofeng Luo, Yujia Dai, Baoshan Xian, Jiahui Xu, Ranran Zhang, Muhammad Saad Rehmani, Chuan Zheng, Xiaoting Zhao, Kaitao Mao, Xiaotong Ren, Shaowei Wei, Lei Wang, Juan He, Weiming Tan, Junbo Du, Weiguo Liu, Shu Yuan and Kai Shu.
Journal of Integrative Plant Biology (2024) Abstract: "Transcriptional regulation plays a key role in the control of seed dormancy, and many transcription factors (TFs) have been documented. However, the mechanisms underlying the interactions between different TFs within a transcriptional complex regulating seed dormancy remain largely unknown. Here, we showed that TF PHYTOCHROME-INTERACTING FACTOR4 (PIF4) physically interacted with the abscisic acid (ABA) signaling responsive TF ABSCISIC ACID INSENSITIVE4 (ABI4) to act as a transcriptional complex to promote ABA biosynthesis and signaling, finally deepening primary seed dormancy. Both pif4 and abi4 single mutants exhibited a decreased primary seed dormancy phenotype, with a synergistic effect in the pif4/abi4 double mutant. PIF4 binds to ABI4 to form a heterodimer, and ABI4 stabilizes PIF4 at the protein level, whereas PIF4 does not affect the protein stabilization of ABI4. Subsequently, both TFs independently and synergistically promoted the expression of ABI4 and NCED6, a key gene for ABA anabolism. The genetic evidence is also consistent with the phenotypic, physiological and biochemical analysis results. Altogether, this study revealed a transcriptional regulatory cascade in which the PIF4–ABI4 transcriptional activator complex synergistically enhanced seed dormancy by facilitating ABA biosynthesis and signaling."
Julio Retamales's insight:
Good contribution!
From
doi
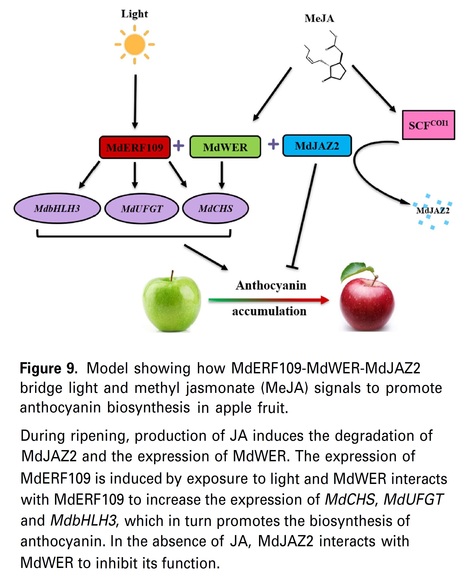
Authors: Xi Zhang, Lujia Yu, Mengjiao Zhang, Ting Wu, Tingting Song, Yuncong Yao, Jie Zhang and Ji Tian. The Plant Journal (2024) Significance Statement: Our findings validate a role for the MdERF109-MdWER-MdJAZ2 module in anthocyanin biosynthesis and uncover a novel mechanism for how light and MeJA signals are coordinated anthocyanin biosynthesis in apple fruit. Abstract: "Anthocyanin generation in apples (Malus domestica) and the pigmentation that results from it may be caused by irradiation and through administration of methyl jasmonate (MeJA). However, their regulatory interrelationships associated with fruit coloration are not well defined. To determine whether MdERF109, a transcription factor (TF) involved in light-mediated coloration and anthocyanin biosynthesis, has synergistic effects with other proteins, we performed a yeast two-hybrid assessment and identified another TF, MdWER. MdWER was induced by MeJA treatment, and although overexpression of MdWER alone did not promote anthocyanin accumulation co-overexpression with MdERF109 resulted in significantly increase in anthocyanin biosynthesis. MdWER may form a protein complex with MdERF109 to promote anthocyanin accumulation by enhancing combinations between the proteins and their corresponding genes. In addition, MdWER, as a MeJA responsive protein, interacts with the anthocyanin repressor MdJAZ2. Transient co-expression in apple fruit and protein interaction assays allowed us to conclude that MdERF109 and MdJAZ2 interact with MdWER and take part in the production of anthocyanins upon MeJA treatment and irradiation. Our findings validate a role for the MdERF109-MdWER-MdJAZ2 module in anthocyanin biosynthesis and uncover a novel mechanism for how light and MeJA signals are coordinated anthocyanin biosynthesis in apple fruit."
Authors: Seoyeon Cha, Wang Ki Min and Hak Soo Seo Communications Biology (2024) One-sentence summary: Arabidopsis E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) suppresses the activity of plasma membrane H+-ATPase, thereby maintaining proper pH profiles of the guard cells and subsequent stomatal responses. Abstract: "Plants rely on precise regulation of their stomatal pores to effectively carry out photosynthesis while managing water status. The Arabidopsis CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), a critical light signaling repressor, is known to repress stomatal opening, but the exact cellular mechanisms remain unknown. Here, we show that COP1 regulates stomatal movement by controlling the pH levels in guard cells. cop1-4 mutants have larger stomatal apertures and disrupted pH dynamics within guard cells, characterized by increased vacuolar and cytosolic pH and reduced apoplastic pH, leading to abnormal stomatal responses. The altered pH profiles are attributed to the increased plasma membrane (PM) H+-ATPase activity of cop1-4 mutants. Moreover, cop1-4 mutants resist to growth defect caused by alkali stress posed on roots. Overall, our study highlights the crucial role of COP1 in maintaining pH homeostasis of guard cells by regulating PM H+-ATPase activity, and demonstrates how proton movement affects stomatal movement and plant growth."
Julio Retamales's insight:
Relevant finding!
From
doi
Authors: Run Han, Liang Ma, William Terzaghi, Yan Guo and Jigang Li.
The Plant Journal (2024) Significance Statement: This review summarizes recent major advances in our understanding of how plants coordinately respond to shade and environmental stresses, and discusses the important questions for future research. Abstract: "Shade avoidance syndrome (SAS) is triggered by a low ratio of red (R) to far-red (FR) light (R/FR ratio), which is caused by neighbor detection and/or canopy shade. In order to compete for the limited light, plants elongate hypocotyls and petioles by deactivating phytochrome B (phyB), a major R light photoreceptor, thus releasing its inhibition of the growth-promoting transcription factors PHYTOCHROME-INTERACTING FACTORs. Under natural conditions, plants must cope with abiotic stresses such as drought, soil salinity, and extreme temperatures, and biotic stresses such as pathogens and pests. Plants have evolved sophisticated mechanisms to simultaneously deal with multiple environmental stresses. In this review, we will summarize recent major advances in our understanding of how plants coordinately respond to shade and environmental stresses, and will also discuss the important questions for future research. A deep understanding of how plants synergistically respond to shade together with abiotic and biotic stresses will facilitate the design and breeding of new crop varieties with enhanced tolerance to high-density planting and environmental stresses."
From
doi
Authors: Yan Yan, Haofei Luo, Yuwei Qin, Tingting Yan, Jinbu Jia, Yifeng Hou, Zhijian Liu, Jixian Zhai, Yanping Long, Xian Deng and Xiaofeng Cao.
PNAS (2024) Significance: Photomorphogenesis is a pivotal stage in early seedling growth that begins when the shoot first emerges from the soil and is exposed to light. Here, we investigated the role of post-transcriptional splicing (PTS) in this developmental stage. By applying Nanopore sequencing of full-length nascent RNA, we found that thousands of genes undergo light-responsive PTS. We used snRNA-seq to characterize seedlings under continuous darkness, or after 1 or 6 h of light exposure, revealing that transcripts showing light-responsive PTS are specifically enriched in mesophyll cells. We identified the splicing factor AtPRMT5 and the E3 ubiquitin ligase COP1 as key regulators of these light-responsive PTS events. These findings uncover how cell type–specific regulation of PTS is essential for the onset of photomorphogenesis. Abstract: "Light plays a central role in plant growth and development, providing an energy source and governing various aspects of plant morphology. Previous study showed that many polyadenylated full-length RNA molecules within the nucleus contain unspliced introns (post-transcriptionally spliced introns, PTS introns), which may play a role in rapidly responding to changes in environmental signals. However, the mechanism underlying post-transcriptional regulation during initial light exposure of young, etiolated seedlings remains elusive. In this study, we used FLEP-seq2, a Nanopore-based sequencing technique, to analyze nuclear RNAs in Arabidopsis (Arabidopsis thaliana) seedlings under different light conditions and found numerous light-responsive PTS introns. We also used single-nucleus RNA sequencing (snRNA-seq) to profile transcripts in single nucleus and investigate the distribution of light-responsive PTS introns across distinct cell types. We established that light-induced PTS introns are predominant in mesophyll cells during seedling de-etiolation following exposure of etiolated seedlings to light. We further demonstrated the involvement of the splicing-related factor A. thaliana PROTEIN ARGININE METHYLTRANSFERASE 5 (AtPRMT5), working in concert with the E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), a critical repressor of light signaling pathways. We showed that these two proteins orchestrate light-induced PTS events in mesophyll cells and facilitate chloroplast development, photosynthesis, and morphogenesis in response to ever-changing light conditions. These findings provide crucial insights into the intricate mechanisms underlying plant acclimation to light at the cell-type level."
Authors: Zhuang Li, Xiangguang Lyu, Hongyu Li, Qichao Tu, Tao Zhao, Jun Liu and Bin Liu. Nature Communications (2024) Editor's view: This study provides insights into how shade induces leaf senescence in soybean. The reduction of blue light intensity deactivates GmCRY1s, leading to the degradation of GmRGAs and the upregulation of WRKY100, ultimately promoting leaf senescence. Abstract: "Leaf senescence is a crucial trait that has a significant impact on crop quality and yield. Previous studies have demonstrated that light is a key factor in modulating the senescence process. However, the precise mechanism by which plants sense light and control senescence remains largely unknown, particularly in crop species. In this study, we reveal that the reduction in blue light under shading conditions can efficiently induce leaf senescence in soybean. The blue light receptors GmCRY1s rather than GmCRY2s, primarily regulate leaf senescence in response to blue light signals. Our results show that GmCRY1s interact with DELLA proteins under light-activated conditions, stabilizing them and consequently suppressing the transcription of GmWRKY100 to delay senescence. Conversely, LBL reduces the interaction between GmCRY1s and the DELLA proteins, leading to their degradation and premature senescence of leaves. Our findings suggest a GmCRY1s-GmDELLAs-GmWRKY100 regulatory cascade that is involved in mediating LBL-induced leaf senescence in soybean, providing insight into the mechanism of how light signals regulate leaf senescence. Additionally, we generate GmWRKY100 knockout soybeans that show delayed leaf senescence and improved yield under natural field conditions, indicating potential applications in enhancing soybean production by manipulating the leaf senescence trait."
Julio Retamales's insight:
Relevant contribution!
|