Your new post is loading...
 Your new post is loading...
Authors: Jingyi Han (撖静宜), Thomas Welch, Ute Voß, Teva Vernoux, Rahul Bhosale and Anthony Bishopp.
iScience (2024)
Highlights • The first intron of ARF7 is required for expression in the root apical meristem. • It is not required for expression in either lateral roots or the shoot apex. • We find no binding sites in the first intron required for expression in root tips. • We propose that this intron exerts its effects via Intron Mediated Enhancement.
Abstract: "Auxin regulates plant growth and development through the transcription factors of the AUXIN RESPONSE FACTOR (ARF) gene family. ARF7 is one of five activators that bind DNA and elicit downstream transcriptional responses. In roots, ARF7 regulates growth, gravitropism and redundantly with ARF19, lateral root organogenesis. In this study we analyzed ARF7 cis-regulation, using different non-coding sequences of the ARF7 locus to drive GFP. We show that constructs containing the first intron led to increased signal in the root tip. Although bioinformatics analyses predicted several transcription factor binding sites in the first intron, we were unable to significantly alter expression of GFP in the root by mutating these. We instead observed the intronic sequences needed to be present within the transcribed sequences to drive expression in the root meristem. These data support a mechanism by which Intron Mediated Enhancement regulates the tissue specific expression of ARF7 in the root meristem."
Authors: Pengcheng Li, Zhihai Zhang, Gui Xiao, Zheng Zhao, Kunhui He, Xiaohong Yang, Qingchun Pan, Guohua Mi, Zhongtao Jia, Jianbing Yan, Fanjun Chen and Lixing Yuan.
Theoretical and Applied Genetics (2024)
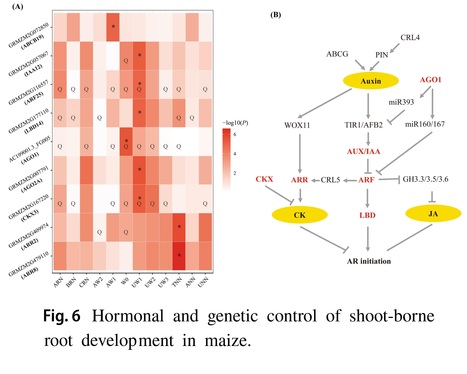
Abstract: "Efficient nutrient and water acquisition from soils depends on the root system architecture (RSA). However, the genetic determinants underlying RSA in maize remain largely unexplored. In this study, we conducted a comprehensive genetic analysis for 14 shoot-borne root traits using 513 inbred lines and 800 individuals from four recombinant inbred line (RIL) populations at the mature stage across multiple field trails. Our analysis revealed substantial phenotypic variation for these 14 root traits, with a total of 389 and 344 QTLs identified through genome-wide association analysis (GWAS) and linkage analysis, respectively. These QTLs collectively explained 32.2–65.0% and 23.7–63.4% of the trait variation within each population. Several a priori candidate genes involved in auxin and cytokinin signaling pathways, such as IAA26, ARF2, LBD37 and CKX3, were found to co-localize with these loci. In addition, a total of 69 transcription factors (TFs) from 27 TF families (MYB, NAC, bZIP, bHLH and WRKY) were found for shoot-borne root traits. A total of 19 genes including PIN3, LBD15, IAA32, IAA38 and ARR12 and 19 GWAS signals were overlapped with selective sweeps. Further, significant additive effects were found for root traits, and pyramiding the favorable alleles could enhance maize root development. These findings could contribute to understand the genetic basis of root development and evolution, and provided an important genetic resource for the genetic improvement of root traits in maize."
Via Jean-Michel Ané
Authors: Yiran Xu, Shengdong Qi, Yong Wang and Jingbo Jia.
Journal of Experimental Botany (2024)
One-sentence summary: This review summarizes the interplay between nitrate and abscisic acid and provides research perspectives for breeding high nitrogen use efficiency and stress-tolerant crop varieties.
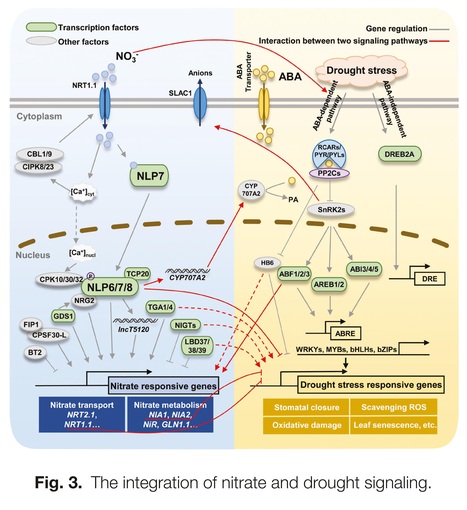
Abstract: "To meet the demands of the new Green Revolution and sustainable agriculture, it is important to develop crop varieties with improved yield, nitrogen use efficiency, and stress resistance. Nitrate is the major form of inorganic nitrogen available for plant growth in many well-aerated agricultural soils, and acts as a signaling molecule regulating plant development, growth, and stress responses. Abscisic acid (ABA), an important phytohormone, plays vital roles in integrating extrinsic and intrinsic responses and mediating plant growth and development in response to biotic and abiotic stresses. Therefore, elucidating the interplay between nitrate and ABA can contribute to crop breeding and sustainable agriculture. Here, we review studies that have investigated the interplay between nitrate and ABA in root growth modulation, nitrate and ABA transport processes, seed germination regulation, and drought responses. We also focus on nitrate and ABA interplay in several reported omics analyses with some important nodes in the crosstalk between nitrate and ABA. Through these insights, we proposed some research perspectives that could help to develop crop varieties adapted to a changing environment and to improve crop yield with high nitrogen use efficiency and strong stress resistance."
Authors: Andrés Rico-Medina, David Blasco-Escámez, Juan B. Fontanet-Manzaneque, Natalie Laibach, Fidel Lozano-Elena, Damiano Martignago and Ana I. Caño-Delgado.
bioRxiv (2024)
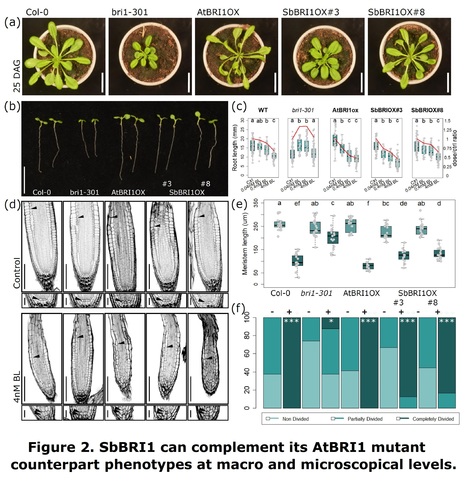
Abstract: "- The high sequence and structural similarities between BRI1 brassinosteroid receptors of Arabidopsis (AtBRI1) and sorghum (SbBRI1) prompted us to study the functionally conserved roles of BRI in both organisms. - Introducing sorghum SbBRI1 in Arabidopsis bri1 mutants restores defective growth and developmental phenotypes to WT levels. - Sorghum mutants for SbBRI1 receptors show defective BR sensitivity and impaired growth and development throughout the entire sorghum life cycle. Embryonic analysis of sorghum primary roots permit to trace back root growth and development to early stages, revealing the functionally conserved roles of SbBRI1 receptor in BR perception during meristem development. RNA-seq analysis uncovers the downstream regulation of the SbBRI1 pathway in cell wall biogenesis during cell growth. - Together, these results uncover that sorghum SbBRI1 receptor protein play functionally conserved roles in plant growth and development, while encourage the study of BR pathways in sorghum and its implications for improving resilience in cereal crops."
Authors: Yewei Zhou, Chunyan Wang, Yongqiang Yu, Zhaojun Ding and Tongda Xu.
The Innovation Life (2024)
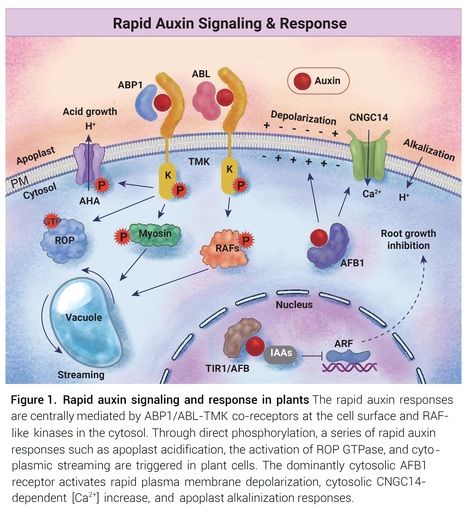
Abstract: "Rapid auxin responses in plants are crucial in initiating cellular changes. These responses are involved in processes such as plasma membrane depolarization, cytoplasmic streaming, apoplastic pH changes, calcium influx, etc. Recent studies illustrated how auxin triggers rapid changes in protein phosphorylation in different species through both the ABP-TMK auxin perception at the cell surface and a conserved RAF-like kinase-based mechanism. These works uncovered an ancient system for rapid responses to the auxin signaling molecule, shedding light on its profound impact on various cellular pathways and functions."
Authors: Renata P. Pincelli-Souza, Qian Tang, Brandon M. Miller and Jerry D. Cohen.
Horticultural Advances (2024)
Abstract: "The first reports that auxins promoted root formation in cuttings and that indole-3-butyric acid (IBA) was a particularly effective treatment date from the early 1930s. Since its introduction into horticultural practice, the focus on improvements in the rooting of plants has been largely on the proper use of auxins to enhance adventitious rooting (AR) as well as to increase the range of plants where it can be effective. In this review, we focus on new ideas that might build on what is known about auxin induction of AR. We explore what the evolution in chemical biology has opened through novel high-throughput screening tools to explore auxin regulation of plant development and what it might add to our understanding and potential to produce new tools for the manipulation of AR. The potential for using stronger auxin analogues, alternative indolealkanoic acids, compounds that alter β-oxidation of IBA and other indolealkanoic acids, auxin conjugates, inhibitors of auxin conjugation, inhibitors of endogenous auxin biosynthesis, as well as other plant hormones and compounds that inhibit the production or mimic the effects of signals that might be involved in AR are all discussed. The expectation is that a summary of these advances in our understanding of the chemical biology important to AR might increase the use and exploration of new ideas for the improvement in the practical approaches to advance horticultural rooting methods."
Authors: Jian You Wang, Guan-Ting Erica Chen, Justine Braguy and Salim Al-Babili.
Trends in Plant Science (2024)
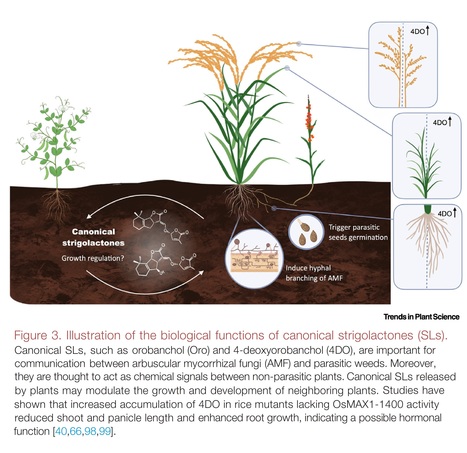
Highlights: Strigolactones (SLs) are structurally diverse and divided into canonical and non-canonical subgroups. SLs are generally considered as plant hormones, best known for inhibiting shoot branching/tillering. SLs are rhizospheric signals important for arbuscular mycorrhizal symbiosis, which may be their ancestral function that is conserved from liverworts to flowering plants. Recent results have revealed functional specificity, indicating that canonical SLs are not the tillering/branching inhibitory hormone in rice or tomato. Increasing the content of 4-deoxyorobanchol in rice by interrupting its hydroxylation affects root, shoot, and panicle growth, suggesting that this canonical SL has hormonal functions. Reducing the levels of canonical SLs by genome editing or applying specific inhibitors is a promising strategy for reducing Striga parasitism.
Abstract: "Strigolactones (SLs) act as regulators of plant architecture as well as signals in rhizospheric communications. Reduced availability of minerals, particularly phosphorus, leads to an increase in the formation and release of SLs that enable adaptation of root and shoot architecture to nutrient limitation and, simultaneously, attract arbuscular mycorrhizal fungi (AMF) for establishing beneficial symbiosis. Based on their chemical structure, SLs are designated as either canonical or non-canonical; however, the question of whether the two classes are also distinguished in their biological functions remained largely elusive until recently. In this review we summarize the latest advances in SL biosynthesis and highlight new findings pointing to rhizospheric signaling as the major function of canonical SLs."
Authors: Qin Zheng, Zhenning Teng, Jianhua Zhang and Nenghui Ye.
Plants (2024)
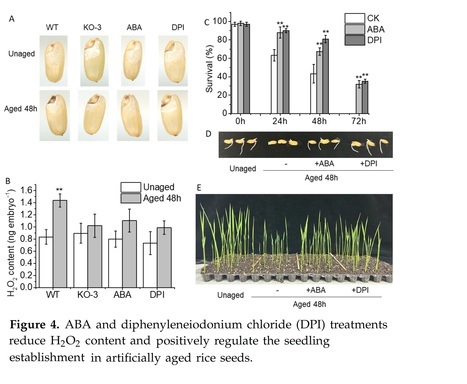
Abstract: "The seed, a critical organ in higher plants, serves as a primary determinant of agricultural productivity, with its quality directly influencing crop yield. Improper storage conditions can diminish seed vigor, adversely affecting seed germination and seedling establishment. Therefore, understanding the seed-aging process and exploring strategies to enhance seed-aging resistance are paramount. In this study, we observed that seed aging during storage leads to a decline in seed vigor and can coincide with the accumulation of hydrogen peroxide (H2O2) in the radicle, resulting in compromised or uneven germination and asynchronous seedling emergence. We identified the abscisic acid (ABA) catabolism gene, abscisic acid 8′-hydroxylase 2 (OsABA8ox2), as significantly induced by aging treatment. Interestingly, transgenic seeds overexpressing OsABA8ox2 exhibited reduced seed vigor, while gene knockout enhanced seed vigor, suggesting its role as a negative regulator. Similarly, seeds pretreated with ABA or diphenyleneiodonium chloride (DPI, an H2O2 inhibitor) showed increased resistance to aging, with more robust early seedling establishment. Both OsABA8ox2 mutant seeds and seeds pretreated with ABA or DPI displayed lower H2O2 content during aging treatment. Overall, our findings indicate that ABA mitigates rice seed aging by reducing H2O2 accumulation in the radicle. This study offers valuable germplasm resources and presents a novel approach to enhancing seed resistance against aging."
Author: Héctor H Torres-Martínez
Plant Physiology (2024)
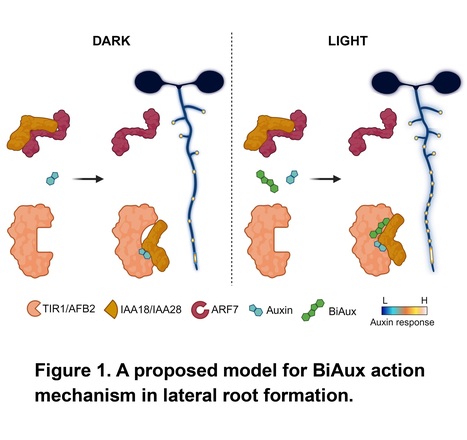
Excerpts: "In this issue of Plant Physiology, González-García et al. (2024) identified a novel compound, BiAux, which is accumulated in roots exposed to light. BiAux binds to a specific site in TIR1/AFBs proteins and, together with auxin, enhances the “sensitivity” to auxin signaling, increasing pre-branch sites along the primary root (Figure 1)."
"In agreement, transcriptomics analysis using BiAux, auxin, and a combined treatment showed that BiAux altered the expression level of genes related to root development and hormone signaling regulated by auxin. Additionally, docking analysis indicated that BiAux binds to a pocket near the auxin binding site in the TIR1 protein. Due to the importance of these residues for TIR1 and Aux/IAA proteins interaction, BiAux is suggested to stabilize the co-receptor formation (Figure 1)."
Authors: Fan Xu, Jia Chen, Yingbin Li, Shilin Ouyang, Mengting Yu, Yirong Wang, Xianming Fang, Kai He and Feng Yu.
Developmental Cell (2024)
Editor's view: Xu and Chen et al. report that the receptor kinase FERONIA interacts with, phosphorylates, and stabilizes the transcription factor PIF3 to regulate PIEZO expression in Arabidopsis root cap, which drives root penetration into soil.
Highlights: • FER participates in Arabidopsis and soybean roots penetration into soil • scRNA-seq profiling of fer-4 root identified PIF3 as a FER-regulated target • FER interacts with, phosphorylates, and stabilizes transcription factor PIF3 • FER-PIF3 module regulates PIEZO expression in Arabidopsis root cap
Abstract: "The cotyledons of etiolated seedlings from terrestrial flowering plants must emerge from the soil surface, while roots must penetrate the soil to ensure plant survival. We show here that the soil emergence-related transcription factor PHYTOCHROME-INTERACTING FACTOR 3 (PIF3) controls root penetration via transducing external signals perceived by the receptor kinase FERONIA (FER) in Arabidopsis thaliana. The loss of FER function in Arabidopsis and soybean (Glycine max) mutants resulted in a severe defect in root penetration into agar medium or hard soil. Single-cell RNA sequencing (scRNA-seq) profiling of Arabidopsis roots identified a distinct cell clustering pattern, especially for root cap cells, and identified PIF3 as a FER-regulated transcription factor. Biochemical, imaging, and genetic experiments confirmed that PIF3 is required for root penetration into soil. Moreover, FER interacted with and stabilized PIF3 to modulate the expression of mechanosensitive ion channel PIEZO and the sloughing of outer root cap cells."
Authors: Yonatan Wexler, Julian I. Schroeder and Doron Shkolnik.
The Plant Journal (2024)
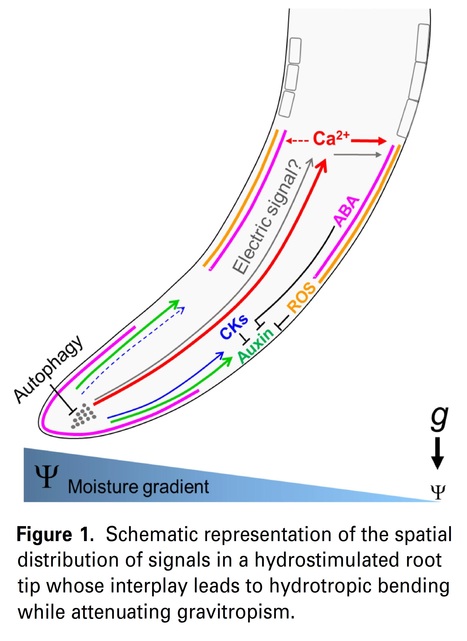
Significance Statement: Understanding the mechanisms that plant roots utilize to resist the strong tendency to grow downward in order to grow toward a moisture source, holds great potential for unveiling research approaches and technologies to improve crop performance. Herein, we assess the complex interplay between major regulators of root growth, development, and responses to myriad internal and external cues, that function to attenuate downward root growth, thereby enabling the root to reach water.
Abstract: "Plants partly optimize their water recruitment from the growth medium by directing root growth toward a moisture source, a phenomenon termed hydrotropism. The default mechanism of downward growth, termed gravitropism, often functions to counteract hydrotropism when the water-potential gradient deviates from the gravity vector. This review addresses the identity of the root sites in which hydrotropism-regulating factors function to attenuate gravitropism and the interplay between these various factors. In this context, the function of hormones, including auxin, abscisic acid, and cytokinins, as well as secondary messengers, calcium ions, and reactive oxygen species in the conflict between these two opposing tropisms is discussed. We have assembled the available data on the effects of various chemicals and genetic backgrounds on both gravitropism and hydrotropism, to provide an up-to-date perspective on the interactions that dictate the orientation of root tip growth. We specify the relevant open questions for future research. Broadening our understanding of root mechanisms of water recruitment holds great potential for providing advanced approaches and technologies that can improve crop plant performance under less-than-optimal conditions, in light of predicted frequent and prolonged drought periods due to global climate change."
Authors: Long Lu, Xinyu Chen, Qinyan Tan, Wenqian Li, Yanyan Sun, Zaoli Zhang, Yuanyuan Song and Rensen Zeng.
Plants (2024)
Abstract: "Aluminum toxicity poses a significant constraint on crop production in acidic soils. While phytohormones are recognized for their pivotal role in mediating plant responses to aluminum stress, the specific involvement of gibberellin (GA) in regulating aluminum tolerance remains unexplored. In this study, we demonstrate that external GA exacerbates the inhibitory impact of aluminum stress on root growth of rice seedlings, concurrently promoting reactive oxygen species (ROS) accumulation. Furthermore, rice plants overexpressing the GA synthesis gene SD1 exhibit enhanced sensitivity to aluminum stress. In contrast, the slr1 gain-of-function mutant, characterized by impeded GA signaling, displays enhanced tolerance to aluminum stress, suggesting the negative regulatory role of GA in rice resistance to aluminum-induced toxicity. We also reveal that GA application suppresses the expression of crucial aluminum tolerance genes in rice, including Al resistance transcription factor 1 (ART1), Nramp aluminum transporter 1 (OsNramp4), and Sensitive to Aluminum 1 (SAL1). Conversely, the slr1 mutant exhibits up-regulated expression of these genes compared to the wild type. In summary, our results shed light on the inhibitory effect of GA in rice resistance to aluminum stress, contributing to a theoretical foundation for unraveling the intricate mechanisms of plant hormones in regulating aluminum tolerance."
Authors: Dipan Roy, Poonam Mehra, Vaishnavi Mukkawar, Lisa Clark, Kevin Bellande, Joop E. M. Vermeer, Raquel Martin Arevallilo, Teva Vernoux, Kawinnat Sue ob, Andrew Jones, Ulrike Bechtold, Phil Mullineaux, Kathryn Lilley, Adrian Brown, Malcolm Bennett and Ari Sadanandom.
bioRxiv (2024)
Abstract: "Reactive oxygen species (ROS) function as key signals in plants to enable adaptation to environmental stresses. Plant roots respond to transient water stress by temporarily ceasing branching using the acclimative response xerobranching1. In this study, we report that a rapid ROS burst regulates Xerobranching by inducing multimerization of auxin repressor protein IAA3/SHY2. Mutations in specific cysteine residues in IAA3/SHY2 disrupt redox-mediated multimerization and interaction with co-repressor TPL, but not with auxin response partner ARF7 and auxin receptor TIR1. ROS-mediated oligomerization of IAA3/SHY2 is required for efficient ARF mediated target gene repression during Xerobranching and lateral root emergence. We demonstrate that AUX/IAA proteins vary in their redox mediated multimerization, revealing a new auxin response regulatory mechanism that directly connects ROS sensing to auxin signalling. Our study reveals how ROS, auxin and water stress intersect to shape acclimative responses in plant roots and maintain their phenotypic plasticity.
|
Authors: Mahamud Hossain Al-Mamun, Christopher Ian Cazzonelli and Priti Krishna.
Frontiers in Plant Science (2024)
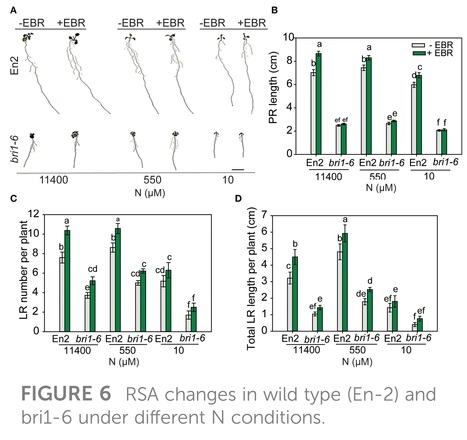
Abstract: "Plants modify their root system architecture (RSA) in response to nitrogen (N) deficiency. The plant steroidal hormone, brassinosteroid (BR), plays important roles in root growth and development. This study demonstrates that optimal levels of exogenous BR impact significant increases in lateral root length and numbers in Arabidopsis seedlings under mild N-deficient conditions as compared to untreated seedlings. The impact of BR on RSA was stronger under mild N deficiency than under N-sufficient conditions. The BR effects on RSA were mimicked in dominant mutants of BZR1 and BES1 (bzr1-1D and bes1-D) transcription factors, while the RSA was highly reduced in the BR-insensitive mutant bri1-6, confirming that BR signaling is essential for the development of RSA under both N-sufficient and N-deficient conditions. Exogenous BR and constitutive activity of BZR1 and BES1 in dominant mutants led to enhanced root meristem, meristematic cell number, and cortical cell length. Under mild N deficiency, bzr1-1D displayed higher fresh and dry shoot weights, chlorophyll content, and N levels in the shoot, as compared to the wild type. These results indicate that BR modulates RSA under both N-sufficient and N-deficient conditions via the transcription factors BES1/BZR1 module and confers tolerance to N deficiency."
Authors: Ping Yun, Cengiz Kaya and Sergey Shabala.
The Crop Journal (2024)
Abstract: "Salinity stress is a major environmental stress affecting crop productivity, and its negative impact on global food security is only going to increase, due to current climate trends. Salinity tolerance was present in wild crop relatives but significantly weakened during domestication. Regaining it back requires a good understanding of molecular mechanisms and traits involved in control of plant ionic and ROS homeostasis. This review summarizes our current knowledge on the role of major plant hormones (auxin, cytokinins, abscisic acid, salicylic acid, and jasmonate) in plants adaptation to soil salinity. We firstly discuss the role of hormones in controlling root tropisms, root growth and architecture (primary root elongation, meristematic activity, lateral root development, and root hairs formation). Hormone-mediated control of uptake and sequestration of key inorganic ions (sodium, potassium, and calcium) is then discussed followed by regulation of cell redox balance and ROS signaling in salt-stressed roots. Finally, the role of epigenetic alterations such as DNA methylation and histone modifications in control of plant ion and ROS homeostasis and signaling is discussed. This data may help develop novel strategies for breeding and cultivating salt-tolerant crops and improving agricultural productivity in saline regions."
Authors: María Isabel Puga, César Poza-Carrión, Iris Martinez-Hevia, Laura Perez-Liens and Javier Paz-Ares
Journal of Plant Research (2024)
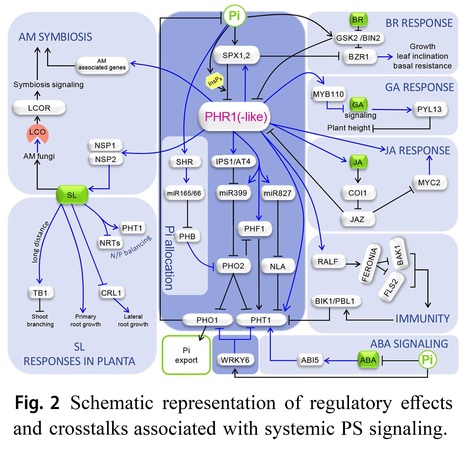
Abstract: "Phosphorus is indispensable for plant growth and development, with its status crucial for determining crop productivity. Plants have evolved various biochemical, morphological, and developmental responses to thrive under conditions of low P availability, as inorganic phosphate (Pi), the primary form of P uptake, is often insoluble in soils. Over the past 25 years, extensive research has focused on understanding these responses, collectively forming the Pi starvation response system. This effort has not only expanded our knowledge of strategies to cope with Pi starvation (PS) but also confirmed their adaptive significance. Moreover, it has identified and characterized numerous components of the intricate regulatory network governing P homeostasis. This review emphasizes recent advances in PS signaling, particularly highlighting the physiological importance of local PS signaling in inhibiting primary root growth and uncovering the role of TORC1 signaling in this process. Additionally, advancements in understanding shoot-root Pi allocation and a novel technique for studying Pi distribution in plants are discussed. Furthermore, emerging data on the regulation of plant-microorganism interactions by the PS regulatory system, crosstalk between the signaling pathways of phosphate starvation, phytohormones and immunity, and recent studies on natural variation in Pi homeostasis are addressed.
Authors: Xiuzhen Kong, Suhang Yu, Yali Xiong, Xiaoyun Song, Lucia Nevescanin-Moreno, Xiaoqing Wei, Jinliang Rao, Hu Zhou, Malcolm J. Bennett, Bipin K. Pandey and Guoqiang Huang.
Current Biology (2024)
Editor's view: Kong et al. provide evidence for the anchorage functions of root hairs in root penetration, a process that is regulated by OsYUC8-mediated auxin biosynthesis and OsAUX1-mediated auxin transport when roots encounter a compacted barrier.
Highlights • Mechanical impedance induced the upregulation of auxin biosynthesis in root apex • Auxin transport from root tip to hair zone promoted root penetration via root hair • Mutants of OsYUC8 and OsAUX1 had trouble penetrating into the compacted layer • Increased root hair length provided greater anchorage force for root penetration
Abstract: "Compacted soil layers adversely affect rooting depth and access to deeper nutrient and water resources, thereby impacting climate resilience of crop production and global food security. Root hair plays well-known roles in facilitating water and nutrient acquisition. Here, we report that root hair also contributes to root penetration into compacted layers. We demonstrate that longer root hair, induced by elevated auxin response during a root compaction response, improves the ability of rice roots to penetrate harder layers. This compaction-induced auxin response in the root hair zone is dependent on the root apex-expressed auxin synthesis gene OsYUCCA8 (OsYUC8), which is induced by compaction stress. This auxin source for root hair elongation relies on the auxin influx carrier AUXIN RESISTANT 1 (OsAUX1), mobilizing this signal from the root apex to the root hair zone. Mutants disrupting OsYUC8 and OsAUX1 genes exhibit shorter root hairs and weaker penetration ability into harder layers compared with wild type (WT). Root-hair-specific mutants phenocopy these auxin-signaling mutants, as they also exhibit an attenuated root penetration ability. We conclude that compaction stress upregulates OsYUC8-mediated auxin biosynthesis in the root apex, which is subsequently mobilized to the root hair zone by OsAUX1, where auxin promotes root hair elongation, improving anchorage of root tips to their surrounding soil environment and aiding their penetration ability into harder layers."
Authors: Yuwen Zhang, Xingliang Duan, Zhen Wang, Yuanda Lv, Weicong Qi, Lun Li, Le Luo and Wei Xuan.
Biochemical and Biophysical Research Communications (2024)
Highlights • Both synthetic AtCEPs and AtCEP5 overexpression suppress primary root elongation and lateral root formation. • The regulation of CEPs on root development requires their receptors CEPRs. • Molecular evidence reveals that CEPs inhibit root development by modulating auxin and cytokinin signaling.
Abstract: "C-terminally encoded peptides (CEPs) are peptide hormones that function as mobile signals coordinating crucial developmental programs in plants. Previous studies have revealed that CEPs exert negative regulation on root development through interaction with CEP receptors (CEPRs), CEPR DOWNSTREAMs (CEPDs), the cytokinin receptor ARABIDOPSIS HISTIDINE KINASE (AHKs) and the transcriptional repressor Auxin/Indole-3-Acetic Acid (AUX/IAA). However, the precise molecular mechanisms underlying CEPs-mediated regulation of root development via auxin and cytokinin signaling pathways still necessitate further detailed investigation. In this study, we examined prior research and elucidated the underlying molecular mechanisms. The results showed that both synthetic AtCEPs and overexpression of AtCEP5 markedly suppressed primary root elongation and lateral root (LR) formation in Arabidopsis. Molecular biology and genetics elucidated how CEPs inhibit root growth by suppressing auxin signaling while promoting cytokinin signaling. In summary, this study elucidated the inhibitory effects of AtCEPs on Arabidopsis root growth and provided insights into their potential molecular mechanisms, thus enhancing our comprehension of CEP-mediated regulation of plant growth and development."
Author: Gwendolyn K. Kirschner.
The Plant Cell (2024)
Excerpts: "Yuxiang Li, Juan Wang and colleagues (Li et al. 2024) now connect the two roles of ethylene: as a signal responding to soil compaction and to trigger crown root development. In this work, they mimicked different levels of compaction by increasing agar concentrations and compared the growth of wildtype plants and ethylene signaling mutants. They found that compaction triggered crown root initiation via an ethylene-dependent pathway (Figure, A), and manipulating ethylene-related regulators influenced both root development and grain yield, implying that these regulators balance both root and grain development."
"By analyzing crown root numbers and transcript levels in oswox11 and ethylene signaling mutants, or overexpression combinations under normal or compacted soil conditions, the authors confirmed that the ethylene-OsEIL1-OsWOX11 module facilitates crown root development in compacted soil (Figure, B)."
Authors: Yuxiang Li, Juan Wang, Yadi Gao, Bipin K Pandey, Lucas León Peralta Ogorek, Yu Zhao, Ruidang Quan, Zihan Zhao, Lei Jiang, Rongfeng Huang and Hua Qin.
The Plant Cell (2024)
One-sentence summary: The phytohormone ethylene fine-tunes rice crown root development by activating WUSCHEL-RELATED HOMEOBOX 11 expression in response to soil compaction.
Abstract: "Optimizing the root architecture of crops is an effective strategy for improving crop yields. Soil compaction is a serious global problem that limits crop productivity by restricting root growth, but the underlying molecular mechanisms are largely unclear. Here, we show that ethylene stimulates rice (Oryza sativa) crown root development in response to soil compaction. First, we demonstrate that compacted soil promotes ethylene production and the accumulation of ETHYLENE INSENSITIVE 3-LIKE 1 (OsEIL1) in rice roots, stimulating crown root primordia initiation and development, thereby increasing crown root number in lower stem nodes. Through transcriptome profiling and molecular analyses, we reveal that OsEIL1 directly activates the expression of WUSCHEL-RELATED HOMEOBOX 11 (OsWOX11), an activator of crown root emergence and growth, and that OsWOX11 mutations delay crown root development, thus impairing the plant’s response to ethylene and soil compaction. Genetic analysis demonstrates that OsWOX11 functions downstream of OsEIL1. In summary, our results demonstrate that the OsEIL1–OsWOX11 module regulates ethylene action during crown root development in response to soil compaction, providing a strategy for the genetic modification of crop root architecture and grain agronomic traits."
Authors: Xiuzhen Kong, Yali Xiong, Xiaoyun Song, Samuel Wadey, Suhang Yu, Jinliang Rao, Aneesh Lale, Marco Lombardi, Riccardo Fusi, Rahul Bhosale and Guoqiang Huang.
Plant Physiology (2024)
Abstract: "Root angle is a critical factor in optimising the acquisition of essential resources from different soil depths. The regulation of root angle relies on the auxin-mediated root gravitropism machinery. While the influence of ethylene on auxin levels is known, its specific role in governing root gravitropism and angle remains uncertain, particularly when Arabidopsis (Arabidopsis thaliana) core ethylene signaling mutants show no gravitropic defects. Our research, focusing on rice (Oryza sativa L.) and maize (Zea mays), clearly reveals the involvement of ethylene in root angle regulation in cereal crops through the modulation of auxin biosynthesis and the root gravitropism machinery. We elucidated the molecular components by which ethylene exerts its regulatory effect on auxin biosynthesis to control root gravitropism machinery. The ethylene-insensitive mutants ethylene insensitive2 (osein2) and ethylene insensitive like1 (oseil1), exhibited substantially shallower crown root angle compared to the wild type. Gravitropism assays revealed reduced root gravitropic response in these mutants. Hormone profiling analysis confirmed decreased auxin levels in the root tips of the osein2 mutant, and exogenous auxin (NAA) application rescued root gravitropism in both ethylene-insensitive mutants. Additionally, the auxin-biosynthetic mutant mao hu zi10 (mhz10)/tryptophan aminotransferase2 (ostar2) showed impaired gravitropic response and shallow crown root angle phenotypes. Similarly, maize ethylene-insensitive mutants (zmein2) exhibited defective gravitropism and root angle phenotypes. In conclusion, our study highlights that ethylene controls the auxin-dependent root gravitropism machinery to regulate root angle in rice and maize, revealing a functional divergence in ethylene signaling between Arabidopsis and cereal crops. These findings contribute to a better understanding of root angle regulation and have implications for improving resource acquisition in agricultural systems.
Authors: Lulu Zheng, Yongfeng Hu, Tianzhao Yang, Zhen Wang, Daoyuan Wang, Letian Jia, Yuanming Xie, Long Luo, Weicong Qi, Yuanda Lv, Tom Beeckman, Wei Xuan and Yi Han.
Nature Communications (2024)
One-sentence summary: This study reports that the SOMBRERO, a root cap-localized transcription factor, determines root halotropic response to salt stress via spatiotemporally modulating AUX1-dependent auxin redistribution in the root tip.
Abstract: "Plants are capable of altering root growth direction to curtail exposure to a saline environment (termed halotropism). The root cap that surrounds root tip meristematic stem cells plays crucial roles in perceiving and responding to environmental stimuli. However, how the root cap mediates root halotropism remains undetermined. Here, we identified a root cap-localized NAC transcription factor, SOMBRERO (SMB), that is required for root halotropism. Its effect on root halotropism is attributable to the establishment of asymmetric auxin distribution in the lateral root cap (LRC) rather than to the alteration of cellular sodium equilibrium or amyloplast statoliths. Furthermore, SMB is essential for basal expression of the auxin influx carrier gene AUX1 in LRC and for auxin redistribution in a spatiotemporally-regulated manner, thereby leading to directional bending of roots away from higher salinity. Our findings uncover an SMB-AUX1-auxin module linking the role of the root cap to the activation of root halotropism."
Authors: Liyu Huang, Yachong Bao, Shiwen Qin, Min Ning, Qinyan Li, Qingmao Li, Shilai Zhang, Guangfu Huang, Jing Zhang, Wensheng Wang, Binying Fu and Fengyi Hu.
The Crop Journal (2024)
Abstract: "Upland rice shows dryland adaptation in the form of a deeper and denser root system and greater drought resistance than its counterpart, irrigated rice. Our previous study revealed a difference in the frequency of the OsNCED2 gene between upland and irrigated populations. A nonsynonymous mutation (C to T, from irrigated to upland rice) may have led to functional variation fixed by artificial selection, but the exact biological function in dryland adaptation is unclear. In this study, transgenic and association analysis indicated that the domesticated fixed mutation caused functional variation in OsNCED2, increasing ABA levels, root development, and drought tolerance in upland rice under dryland conditions. OsNCED2-overexpressing rice showed increased reactive oxygen species-scavenging abilities and transcription levels of many genes functioning in stress response and development that may regulate root development and drought tolerance. OsNCED2T-NILs showed a denser root system and drought resistance, promoting the yield of rice under dryland conditions. OsNCED2T may confer dryland adaptation in upland rice and may find use in breeding dryland-adapted, water-saving rice."
Authors: Priya Voothuluru, Yajun Wu and Robert E. Sharp
The Plant Cell (2024)
Abstract: "Limited water availability is a major environmental factor constraining plant development and crop yields. One of the prominent adaptations of plants to water deficits is the maintenance of root growth that enables sustained access to soil water. Despite early recognition of the adaptive significance of root growth maintenance under water deficits, progress in understanding has been hampered by the inherent complexity of root systems and their interactions with the soil environment. We highlight selected milestones in understanding of root growth responses to water deficits, with emphasis on founding studies that have shaped current knowledge and set the stage for further investigation. We revisit the concept of integrated biophysical and metabolic regulation of plant growth, and utilize this framework to review central growth-regulatory processes occurring within root growth zones under water stress at sub-cellular to organ scales. Key topics include the primary processes of modifications of cell wall yielding properties and osmotic adjustment, as well as regulatory roles of abscisic acid and its interactions with other hormones. We include consideration of long-recognized responses for which detailed mechanistic understanding has been elusive until recently, for example hydrotropism, and identify gaps in knowledge, ongoing challenges and opportunities for future research."
Authors: Andrea R. Kohler, Andrew Scheil, Joseph L. Hill, Jr., Jeffrey R. Allen, Jameel M. Al-Haddad, Charity Z. Goeckeritz, Lucia C. Strader, Frank W. Telewski and Courtney A. Hollender.
Plant Physiology (2024)
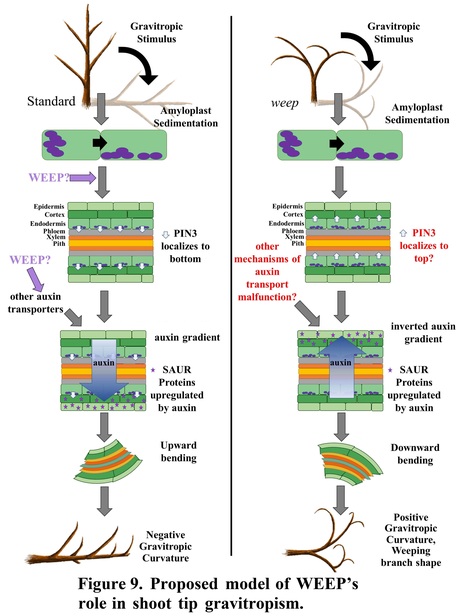
One-sentence summary: Polar auxin transport associated with gravitropism and lateral shoot and root orientation requires the highly conserved WEEP protein in peach.
Abstract: "Trees with weeping shoot architectures are valued for their beauty and are a resource for understanding how plants regulate posture control. The peach (Prunus persica) weeping phenotype, which has elliptical downward arching branches, is caused by a homozygous mutation in the WEEP gene. Little is known about the function of WEEP despite its high conservation throughout Plantae. Here, we present the results of anatomical, biochemical, biomechanical, physiological, and molecular experiments that provide insight into WEEP function. Our data suggest that weeping peach trees do not have defects in branch structure. Rather, transcriptomes from the adaxial (upper) and abaxial (lower) sides of standard and weeping branch shoot tips revealed flipped expression patterns for genes associated with early auxin response, tissue patterning, cell elongation, and tension wood development. This suggests that WEEP promotes polar auxin transport toward the lower side during shoot gravitropic response, leading to cell elongation and tension wood development. In addition, weeping peach trees exhibited steeper root systems and faster lateral root gravitropic response. This suggests that WEEP moderates root gravitropism and is essential to establishing the set-point angle of lateral roots from the gravity vector. Additionally, size-exclusion chromatography indicated that WEEP proteins self-oligomerize, like other proteins with sterile alpha motif (SAM) domains. Collectively, our results from weeping peach provide insight into polar auxin transport mechanisms associated with gravitropism and lateral shoot and root orientation."
|



 Your new post is loading...
Your new post is loading...