Your new post is loading...
 Your new post is loading...
Authors: Dongmei Yan and Huilan Yi.
Scientia Horticulturae (2024)
Highlights • Identifying key genes and pathways for postharvest preservation of grapes by RNA-seq. • SO2 prolongs the postharvest shelf life of table grapes by reducing cell wall degradation and enhancing defenses of the skins. • SO2-triggered secondary metabolism and defense responses in grape fruits contributed to postharvest preservation. • SO2-induced inhibition of ethylene signaling pathway helped to delay postharvest softening and aging of grape fruits.
Abstract: "Sulfur dioxide (SO2) is the most frequently used preservative for table grapes, yet the preservation mechanism remains unclear. To ascertain the specific genes and pathways involved in SO2 preservation, RNA-seq technology was employed to characterize the transcriptome profile of grapes during postharvest storage. A total of 22,288 genes were identified, of which 377 genes were differentially expressed (≥ 2-fold change) between SO2 and control groups, mainly enriched in secondary metabolism, plant-pathogen interactions, plant hormone signaling, etc. Numerous genes encoding pathogenesis-related (PR) proteins exhibited higher expression levels in SO2 group, while the activities of disease-resistant enzymes, such as β-1,3-glucanase (PR2) and chitinase (PR3) significantly increased by 28.9 % and 29.3 % (P < 0.05), indicating SO2-induced plant resistance. Exposure to SO2 also enhanced the expression of genes encoding essential enzymes of secondary metabolism, and significantly increased both the activities of critical enzymes for the biosynthesis of secondary metabolites, such as phenylalanine ammonia-lyase (PAL) and 4-coumarate-CoA ligase (4CL), and the contents of secondary metabolites such as total phenol, flavonoid, anthocyanin, and lignin, demonstrating an enhanced chemical and physical barriers in SO2-fumigated grapes. Meanwhile, the genes associated with ethylene signaling and cell wall degradation were down-regulated by SO2 preservative, and some ethylene-responsive elements were identified in the promoter regions of cell wall hydrolase genes, suggesting that SO2-inhibited ethylene signaling might contribute to maintaining cell wall integrity. Altogether, gene expression patterns and cellular physiological processes were altered in grapes exposed to SO2, which helped maintain fruit quality and prolong postharvest life by regulating the defense responses and fruit ageing."
Authors: Muhmmad Asad Ullah Asad, Zhang Yan, Lujian Zhou, Xianyue Guan and Fangmin Cheng.
Plant Physiology and Biochemistry (2024)
Highlights: • Sugars play an essential role in the regulations of leaf senescence. • Abiotic stresses trigger sugar signaling by inducing reactive oxygen species burst. • Sugar signaling interact with plant hormones and protein kinase to regulates leaf senescence. • Abiotic stresses target sugar signaling to regulate photosynthesis inhibition and programmed cell death (PCD).
Abstract: "Plants have evolved the adaptive capacity to mitigate the negative effect of external adversities at chemical, molecular, cellular, and physiological levels. This capacity is conferred by triggering the coordinated action of internal regulatory factors, in which sugars play an essential role in the regulating chloroplast degradation and leaf senescence under various stresses. In this review, we summarize the recent findings on the senescent-associated changes in carbohydrate metabolism and its relation to chlorophyll degradation, oxidative damage, photosynthesis inhibition, programmed cell death (PCD), and sink-source relation as affected by abiotic stresses. The action of sugar signaling in regulating the initiation and progression of leaf senescence under abiotic stresses involves interactions with various plant hormones, reactive oxygen species (ROS) burst, and protein kinases. This discussion aims to elucidate the complex regulatory network and molecular mechanisms that underline sugar-induced leaf senescence in response to various abiotic stresses. The imperative role of sugar signaling in regulating plant stress responses potentially enables the production of crop plants with modified sugar metabolism. This, in turn, may facilitate the engineering of plants with improved stress responses, optimal life span and higher yield achievement."
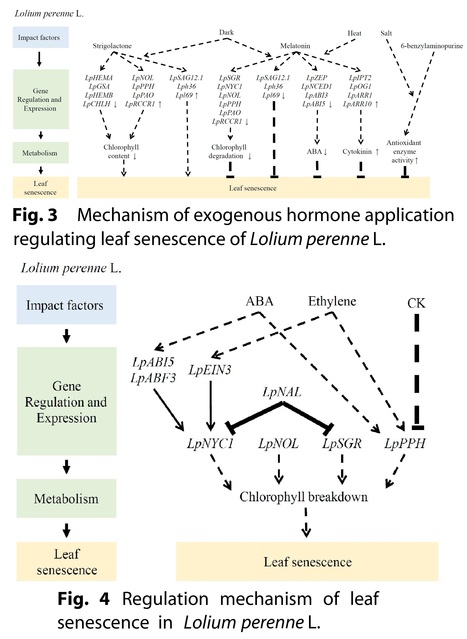
Authors: Kangning Zhang, Hongli Xie, Jiangqi Wen, Jing Zhang, Zeng-Yu Wang, Bin Xu and Maofeng Chai.
Grass Research (2024)
Abstract: "Leaf senescence is a complex biological process regulated by development, phytohormones, and various environmental factors. For forage and turf grasses, controlling leaf senescence can greatly improve forage quality, the amenity of lawn and turf, and the grasses’ stress tolerances. Leaf senescence involves a multitude of gene regulation and metabolic changes, including the alteration of chlorophyll metabolism. Here, we summarized the recent progress of studies on leaf senescence in major forage and turf grass species, such as Medicago truncatula, M. sativa, Lolium perenne, Panicum virgatum, and Agrostis stolonifera, to provide an insight into the development of effective methods for delaying leaf senescence in grass species."
Authors: Wei Chang, Huina Zhao, Hongqiao Chen, Guixiang Jiao, Jing Yu, Bing Wang, Haiqian Xia, Boyu Meng, Xiaodong Li, Mengna Yu, Shengting Li, Mingchao Qian, Yonghai Fan, Kai Zhang, Bo Lei and Kun Lu.
Plant Physiology (2024)
Abstract: "Leaf senescence is a vital aspect of plant physiology and stress responses and is induced by endogenous factors and environmental cues. The plant-specific NAC (NAM, ATAF1/2, CUC2) transcription factor family influences growth, development, and stress responses in Arabidopsis (Arabidopsis thaliana) and other species. However, the roles of NACs in tobacco (Nicotiana tabacum) leaf senescence are still unclear. Here, we report that NtNAC56 regulates leaf senescence in tobacco. Transgenic plants overexpressing NtNAC56 (NtNAC56-OE) showed induction of senescence-related genes and exhibited early senescence and lower chlorophyll content compared to wild-type (WT) plants and the Ntnac56-19 mutant. In addition, root development and seed germination were inhibited in the NtNAC56-OE lines. Transmission electron microscopy observations accompanied by physiological and biochemical assays revealed that NtNAC56 overexpression triggers chloroplast degradation and reactive oxygen species accumulation in tobacco leaves. Transcriptome analysis demonstrated that NtNAC56 activates leaf senescence-related genes and jasmonic acid (JA) biosynthesis pathway genes. In addition, the JA content of NtNAC56-OE plants was higher than in WT plants, and JA treatment induced NtNAC56 expression. We performed DNA affinity purification sequencing to identify direct targets of NtNAC56, among which we focused on LIPOXYGENASE 5 (NtLOX5), a key gene in JA biosynthesis. A dual-luciferase reporter assay and a yeast one-hybrid assay confirmed that NtNAC56 directly binds to the TTTCTT motif in the NtLOX5 promoter. Our results reveal a mechanism whereby NtNAC56 regulates JA-induced leaf senescence in tobacco and provide a strategy for genetically manipulating leaf senescence and plant growth."
Authors: Guoling Guo, Lun Liu, Taijing Shen, Haozhe Wang, Shuqin Zhang, Yu Sun, Guoyu Xiong, Xiaomei Tang, Liwu Zhu and Bing Jia.
BMC Plant Biology (2024)
Abstract: "Background - Chlorophyll (Chl) is an agronomic trait associated with photosynthesis and yield. Gibberellin 2-oxidases (GA2oxs) have previously been shown to be involved in Chl accumulation. However, whether and how the PbrGA2ox proteins (PbrGA2oxs) mediate Chl accumulation in pear (Pyrus spp.) is scarce. Results - Here, we aimed to elucidate the role of the pear GA2ox gene family in Chl accumulation and the related underlying mechanisms. We isolated 13 PbrGA2ox genes (PbrGA2oxs) from the pear database and identified PbrGA2ox1 as a potential regulator of Chl accumulation. We found that transiently overexpressing PbrGA2ox1 in chlorotic pear leaves led to Chl accumulation, and PbrGA2ox1 silencing in normal pear leaves led to Chl degradation, as evident by the regreening and chlorosis phenomenon, respectively. Meanwhile, PbrGA2ox1-overexpressing (OE) tobacco plants discernably exhibited Chl built-up, as evidenced by significantly higher Pn and Fv/Fm. In addition, RNA sequencing (RNA-seq), physiological and biochemical investigations revealed an increase in abscisic acid (ABA), methyl jasmonate (MeJA), and salicylic acid (SA) concentrations and signaling pathways; a marked elevation in reducing and soluble sugar contents; and a marginal decline in the starch and sucrose levels in OE plants. Interestingly, PbrGA2ox1 overexpression did not prominently affect Chl synthesis. However, it indeed facilitated chloroplast development by increasing chloroplast number per cell and compacting the thylakoid granum stacks. These findings might jointly contribute to Chl accumulation in OE plants. Conclusion - Overall, our results suggested that GA2oxs accelerate Chl accumulation by stimulating chloroplast development and proved the potential of PbrGA2ox1 as a candidate gene for genetically breeding biofortified pear plants with a higher yield."
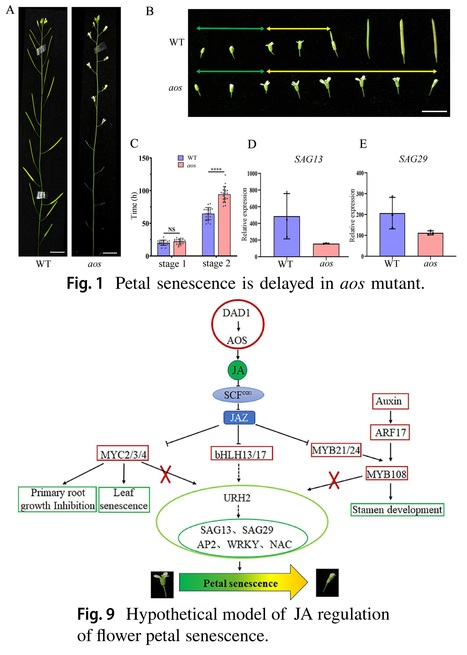
Authors: Liuqing Wu, Kaiqi Wang, Mengyi Chen, Wenxin Su, Zheng Liu, Xiaoying Guo, Mengqian Ma, Shuangjie Qian, Yuqi Deng, Haihan Wang, Chanjuan Mao, Zaibao Zhang and Xiaofeng Xu.
Physiology and Molecular Biology of Plants (2024)
Abstract: "Flowers are crucial for the reproduction of flowering plants and their senescence has drastic effects on plant-animal interactions as well as pollination. Petal senescence is the final phase of flower development which is regulated by hormones and genes. Among these, jasmonic acid (JA) has emerged as a major contributor to petal senescence, but its molecular mechanisms remain elusive. Here, the role of JA in petal senescence in Arabidopsis was investigated. We showed that petal senescence in aos mutant was significantly delayed, which also affected petal cell size and proliferation. Similar significant delays in petal senescence were observed in dad1 and coi1 mutants. However, MYB21/24 and MYC2/3/4, known downstream regulators of JA in flower development, played no role in petal senescence. This indicated that JA regulates petal senescence by modulating other unknown transcription factors. Transcriptomic analysis revealed that AOS altered the expression of 3681 genes associated, and identified groups of differentially expressed transcription factors, highlighting the potential involvement of AP-2, WRKY and NAC. Furthermore, bHLH13, bHLH17 and URH2 were identified as potential new regulators of JA-mediated petal senescence. In conclusion, our findings suggest a novel genetic pathway through which JA regulates petal senescence in Arabidopsis. This pathway operates independently of stamen development and leaf senescence, suggesting the evolution of specialized mechanisms for petal senescence."
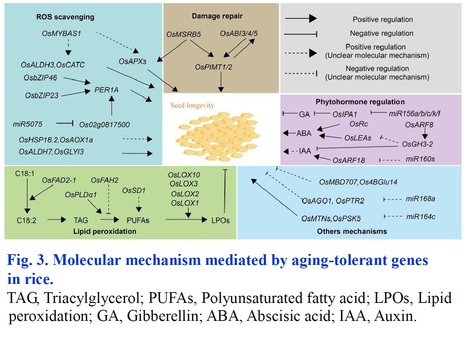
Authors: Tianshun Zhou, Dong Yu, Liubing Wu, Yusheng Xu, Meijuan Duan and Dingyang Yuan.
Rice Science (2024)
Abstract: "Long-term storage of crop seeds is critical for germplasm resource conservation, food supply and sustainable production. As a major food, rice has a huge stock for production and consumption worldwide, while easily losing food value and seed viability during storage. Thus, understanding the physiological responses and molecular mechanisms of aging tolerance lays the foundation for improving seed storability in rice. This review illustrates the current advances in influential factors, evaluation methods, and identification indexes of seed storability. It also discusses the physiological consequences, molecular mechanisms, and methods to breed aging-tolerant rice in detail. Finally, it points out some challenges in the research of seed storability that need to be addressed in the future. This review provides a theoretical basis and research direction for revealing the mechanisms underlying seed storability and breeding aging tolerant rice."
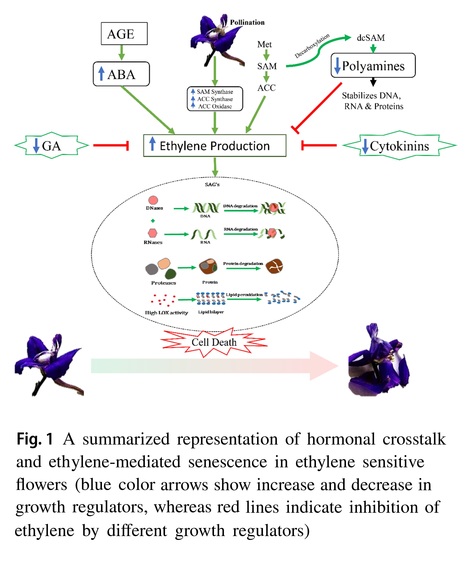
Authors: Aehsan ul Haq, Sumira Farooq, Mohammad Lateef Lone, Shazia Parveen, Foziya Altaf and Inayatullah Tahir
Journal of Plant Growth Regulation (2024)
Abstract: "Postharvest senescence of cut flowers is a stumbling impediment in harnessing their commercial potential. Consequently, the postharvest quality preservation of cut flowers is a crucial factor to allure buyers and maximize economic gains. Flower senescence being final phase of organ development is a key factor triggering postharvest quality deterioration. The process of flower senescence is closely regulated by developmental and environmental cues. The perception of these signals subsequently involves loss of membrane integrity, decreased activity of antioxidant enzymes, and upregulation of proteases and nucleases, which are key signatures of senescence and culminate in the death of petal tissues. Moreover, the developmental and environmental cues are synchronized by considerable turnover in different growth regulators, particularly cytokinins, abscisic acid, ethylene, and gibberellic acid, which act both antagonistically and synergistically to coordinate the senescence process in flowers. Among these growth regulators, ethylene has a crucial role in orchestrating petal senescence in ethylene-responsive systems, while, abscisic acid regulates petal senescence in ethylene-independent systems. Recent research on ethylene-sensitive flowers revealed that the crosstalk of ethylene with sugars and other growth regulators plays a crucial role in modulating senescence by affecting the expression of ethylene-responsive genes. Despite the plethora of postharvest studies conducted so far, considerable miss links still persist in understanding the intricacies of senescence regulating mechanisms, mainly in ethylene-responsive flowers. To this end, it is imperative to critically re-evaluate our current understanding of ethylene-dependent flower senescence to gain intricate inputs regarding the underlying senescence mechanisms, particularly in ornamental families like Ranunculaceae. This constitutes the pivotal gateway toward deciphering the enigmatic complexities governing senescence regulatory mechanisms, thereby forging a path for postharvest researchers to craft pioneering methodologies aimed at accentuating the longevity of commercially significant flowers, thereby yielding substantial economic ramifications."
Authors: Yi Zhang, Yingying Xing, Xinyu Tian, Liuhui Yang, Likai Wang, Zhiyong Guan, Jiafu Jiang, Fadi Chen and Sumei Chen.
Postharvest Biology and Technology (2024)
Highlights: • Sucrose antagonizes SL-induced leaf senescence in chrysanthemum. • Various genes antagonistically regulated by SL and sucrose were revealed by RNA-seq. • CmWRKY6-Like positively regulates leaf senescence in chrysanthemum. • CmWRKY6-Like involves the antagonistic regulation of leaf senescence by SL and sugar.
Abstract: "Strigolactone (SL), a novel plant hormone, plays a vital role in promoting leaf senescence. Sugar, a nutrient source widely used to retain freshness of cut flowers, was recently reported to alleviate SL-induced leaf senescence; however, the underlying molecular mechanisms remain unclear. Leaf senescence during transportation and storage of cut chrysanthemum considerably affects its shelf life and ornamental value. In this study, we found that sucrose antagonizes SL-induced leaf senescence in chrysanthemum. Transcriptional reprogramming analysis revealed a number of differentially expressed genes antagonistically regulated by SL and sucrose, mainly including those related to SL signaling, sugar signaling, ethylene, auxin, jasmonic acid, reactive oxygen species, chlorophyll metabolism pathways, and transcription factors (such as WRKY, NAC, AP2/ERF, and MYB). Virus-based transient silencing of CmWRKY6-Like in chrysanthemum revealed that CmWRKY6-Like positively regulates leaf senescence and involves the antagonistic regulation of leaf senescence by SL and sucrose. This study provides a basis for understanding the molecular mechanisms underlying the antagonistic roles of SL- and sugar-mediated leaf senescence in chrysanthemum."
Authors: Zhuang Li, Xiangguang Lyu, Hongyu Li, Qichao Tu, Tao Zhao, Jun Liu and Bin Liu.
Nature Communications (2024)
Editor's view: This study provides insights into how shade induces leaf senescence in soybean. The reduction of blue light intensity deactivates GmCRY1s, leading to the degradation of GmRGAs and the upregulation of WRKY100, ultimately promoting leaf senescence.
Abstract: "Leaf senescence is a crucial trait that has a significant impact on crop quality and yield. Previous studies have demonstrated that light is a key factor in modulating the senescence process. However, the precise mechanism by which plants sense light and control senescence remains largely unknown, particularly in crop species. In this study, we reveal that the reduction in blue light under shading conditions can efficiently induce leaf senescence in soybean. The blue light receptors GmCRY1s rather than GmCRY2s, primarily regulate leaf senescence in response to blue light signals. Our results show that GmCRY1s interact with DELLA proteins under light-activated conditions, stabilizing them and consequently suppressing the transcription of GmWRKY100 to delay senescence. Conversely, LBL reduces the interaction between GmCRY1s and the DELLA proteins, leading to their degradation and premature senescence of leaves. Our findings suggest a GmCRY1s-GmDELLAs-GmWRKY100 regulatory cascade that is involved in mediating LBL-induced leaf senescence in soybean, providing insight into the mechanism of how light signals regulate leaf senescence. Additionally, we generate GmWRKY100 knockout soybeans that show delayed leaf senescence and improved yield under natural field conditions, indicating potential applications in enhancing soybean production by manipulating the leaf senescence trait."
Authors: Suk-Hwan Kim, Jungwon Yoon, Hanna Kim, Sang-Ji Lee & Nam-Chon Paek.
Rice (2023)
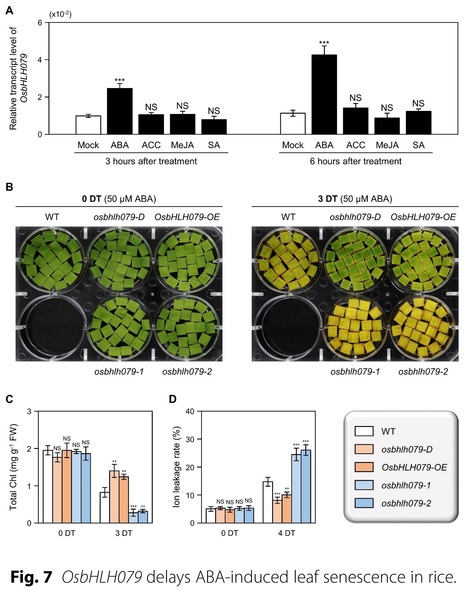
Abstract: "Leaf senescence represents the final phase of leaf development and is characterized by a highly organized degenerative process involving the active translocation of nutrients from senescing leaves to growing tissues or storage organs. To date, a large number of senescence-associated transcription factors (sen-TFs) have been identified that regulate the initiation and progression of leaf senescence. Many of these TFs, including NAC (NAM/ATAF1/2/CUC2), WRKY, and MYB TFs, have been implicated in modulating the expression of downstream senescence-associated genes (SAGs) and chlorophyll degradation genes (CDGs) under the control of phytohormones. However, the involvement of basic helix-loop-helix (bHLH) TFs in leaf senescence has been less investigated. Here, we show that OsbHLH079 delays both natural senescence and dark-induced senescence: Overexpression of OsbHLH079 led to a stay-green phenotype, whereas osbhlh079 knockout mutation displayed accelerated leaf senescence. Similar to other sen-TFs, OsbHLH079 showed a gradual escalation in expression as leaves underwent senescence. During this process, the mRNA levels of SAGs and CDGs remained relatively low in OsbHLH079 overexpressors, but increased sharply in osbhlh079 mutants, suggesting that OsbHLH079 negatively regulates the transcription of SAGs and CDGs under senescence conditions. Additionally, we found that OsbHLH079 delays ABA-induced senescence. Subsequent RT-qPCR and dual-luciferase reporter assays revealed that OsbHLH079 downregulates the expression of ABA signaling genes, such as OsABF2, OsABF4, OsABI5, and OsNAP. Taken together, these results demonstrate that OsbHLH079 functions in delaying leaf yellowing by attenuating the ABA responses."
Authors: Yun-Jing Bao, Jia-Xu Chen, Youjun Zhang, Alisdair R. Fernie, Jianhua Zhang, Bao-Xing Huang, Fu-Yuan Zhu and Fu-Liang Cao.
Advanced Agrochem (2024)
Highlights: • This review addresses the multiple roles served by JA-involved regulatory networks in different woody species, which have similar properties and differential regulation within their herbaceous counterparts, especially focusing on developmental growth. • The potential role of AS a regulatory mechanism of JA modulation, which is concerned with plant development and stress responses. The utilization of proteogenomic analysis would further advance our standing of JA-mediated AS regulation and elucidate the specific mechanisms in woody plants.
Abstract: "Jasmonic acid is a crucial phytohormone that plays a pivotal role, serving as a regulator to balancing plant development and resistance. However, there are analogous and distinctive characteristics exhibited in JA biosynthesis, perception, and signal transduction pathways in both herbaceous and woody plants. Moreover, the majority of research subjects have predominantly focused on the function of JA in model or herbaceous plants. Consequently, there is a significant paucity of studies investigating JA regulation networks in woody plants, particularly concerning post-transcriptional regulatory events such as alternative splicing (AS). This review article aims to conduct a comprehensive summary of advancements that JA signals regulate plant development across various woody species, comparing the analogous features and regulatory differences to herbaceous counterparts. In addition, we summarized the involvement of AS events including splicing factor (SF) and transcripts in the JA regulatory network, highlighting the effectiveness of high-throughput proteogenomic methods. A better understanding of the JA signaling pathway in woody plants has pivotal implications for forestry production, including optimizing plant management and enhancing secondary metabolite production."
Authors: Hanqian Feng, Jinjuan Tan and Zhiping Deng.
Journal of Experimental Botany (2023)
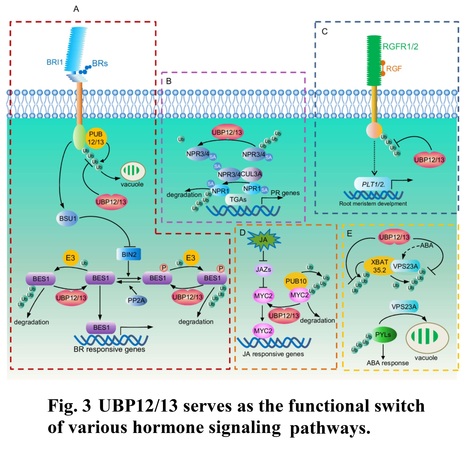
Abstract: "Ubiquitination, a vital post-translational modification in plants, plays a significant role in regulating protein activity, localization, and stability. This process occurs through a complex enzyme cascade that involves E1, E2, and E3 enzymes, leading to the covalent attachment of ubiquitin molecules to substrate proteins. Conversely, deubiquitinating enzymes (DUBs) work in opposition to this process by removing ubiquitin moieties. Despite extensive research on ubiquitination in plants, our understanding of the function of DUBs is still emerging. UBP12 and UBP13, two plant DUBs, have received much attention recently and are shown to play pivotal roles in hormone signaling, light perception, photoperiod responses, leaf development, senescence, and epigenetic transcriptional regulation. This review summarizes current knowledge about these two enzymes, highlighting the central role of deubiquitination in regulating the abundance and activity of critical regulators like receptor kinases and transcriptional factors during phytohormone and developmental signaling."
|
Authors: Juncai Deng, Xiangqing Huang, Jianhua Chen, Bartel Vanholme, Jinya Guo, Yuanyuan He, Wenting Qin, Jing Zhang, Wenyu Yang and Jiang Liu.
Plant Physiology and Biochemistry (2024)
Highlights: • Shade stress leads to premature senescence in soybean plants. • Ethylene biosynthesis and signal transduction are induced to regulate soybean plant senescence. • Shade stress retains nitrogen in soybean vegetative organs and impedes the remobilization of nitrogen from these organs. • These negative impacts can be mitigated by reducing the intensity of shade stress through field layout optimization.
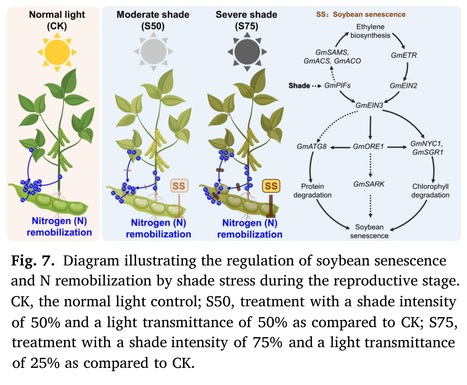
Abstract: "In gramineae-soybean intercropping systems, shade stress caused by taller plants impacts soybean growth specifically during the reproductive stage. However, the effects of shade stress on soybean senescence remain largely unexplored. In this research, we applied artificial shade treatments with intensities of 75% (S75) and 50% (S50) to soybean plants at the onset of flowering to simulate the shade stress experienced by soybeans in the traditional and optimized maize-soybean intercropping systems, respectively. Compared to the normal light control, both shade treatments led to a rapid decline in the dry matter content of soybean vegetative organs and accelerated their abscission. Moreover, shade treatments triggered the degradation of chlorophyll and soluble proteins in leaves and increased the expression of genes associated with leaf senescence. Metabolic profiling further revealed that ethylene biosynthesis and signal transduction were induced by shade treatment. In addition, the examination of nitrogen content demonstrated that shade treatments impeded the remobilization of nitrogen in vegetative tissues, consequently reducing the seed nitrogen harvest. It's worth noting that these negative effects were less pronounced under the S50 treatment compared to the S75 treatment. Taken together, this research demonstrates that shade stress during the reproductive stage accelerates soybean senescence and impedes nitrogen remobilization, while optimizing the field layout to improve soybean growth light conditions could mitigate these challenges in the maize-soybean intercropping system."
Authors: Yexing Jing, Ziyi Yang, Zongju Yang, Wanqing Bai, Ruizhen Yang, Yanjun Zhang, Kewei Zhang, Yunwei Zhang and Jiaqiang Sun.
New Phytologist (2024)
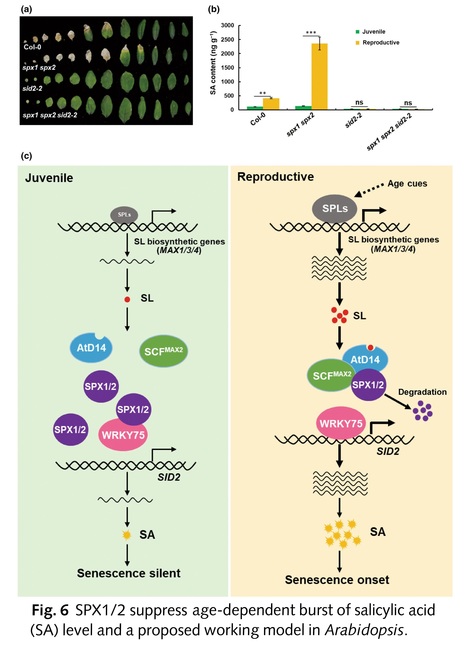
Abstract: "Leaf senescence is a complex process strictly regulated by various external and endogenous factors. However, the key signaling pathway mediating leaf senescence remains unknown. Here, we show that Arabidopsis SPX1/2 negatively regulate leaf senescence genetically downstream of the strigolactone (SL) pathway. We demonstrate that the SL receptor AtD14 and MAX2 mediate the age-dependent degradation of SPX1/2. Intriguingly, we uncover an age-dependent accumulation of SLs in leaves via transcriptional activation of SL biosynthetic genes by the transcription factors (TFs) SPL9/15. Furthermore, we reveal that SPX1/2 interact with the WRKY75 subclade TFs to inhibit their DNA-binding ability and thus repress transcriptional activation of salicylic acid (SA) biosynthetic gene SA Induction-Deficient 2, gating the age-dependent SA accumulation in leaves at the leaf senescence onset stage. Collectively, our new findings reveal a signaling pathway mediating sequential activation of SL and salicylate biosynthesis for the onset of leaf senescence in Arabidopsis."
Author: Pablo Ignacio Calzadilla.
Plant Physiology (2024)
Excerpts: "In this Issue of Plant Physiology, Chang et al. (2024) studied the connection between NAC TFs and JA in regulating leaf senescence in tobacco plants. Exploring previous transcriptome data, the authors identified NtNAC56 as a candidate regulatory TF for leaf senescence. To study NtNAC56’s role, transgenic lines over-expressing NtNAC56 (NtNAC56-OE) were generated and characterized. The NtNAC56-OE lines senesced earlier than the wild type (WT) plants. NtNAC56-OE plants had significantly lower chlorophyll content and photosynthetic capacity and higher expression of SAGs when compared to WT."
"In summary, Chang et al. (2024) used a broad variety of techniques to explore the regulatory mechanisms inducing leaf senescence in tobacco. They identified NtNAC56 as a novel TF contributing to JA-induced leaf senescence. Data showed a positive activation loop between NtNAC56 and JA, and that NtNAC56 directly regulates JA biosynthesis through its binding to the specific TTTCTT cis-regulatory motif (Figure 1). Overall, this study contributes to our knowledge of the complex signaling network regulating leaf senescence."
Authors: Nikita Yadav, Preeti Nagar, Abhilasha Rawat and Ananda Mustafiz.
Environmental and Experimental Botany (2024)
Highlights: • Expression of PSKR is up-regulated in the senescent leaves. • Over-expressing lines of AtPSKR1 and OsPSKR10 show an early leaf senescence phenotype. • AtPSKR1 and OsPSKR10 trigger superoxides accumulation in leaves. • AtPSKR1 and OsPSKR10 interact with the NADPH oxidases (AtRBOHD and AtRBOHF).
Abstract "Leaf senescence represents an active and regulated degeneration process. Leucine-rich repeat receptor-like protein kinase (LRR-RLK) represents a large group of cell surface receptors that play important roles in multiple biological processes, and some are also known to regulate the senescence process. In the present study, we elucidated the involvement of Phytosulfokine Receptor (PSKR) gene in the regulation of leaf senescence. The expression of PSKR1 of Arabidopsis was found to be up-regulated during leaf senescence. The over-expressing lines of AtPSKR1 and its rice homologue OsPSKR10 displayed an early leaf senescent phenotype in Arabidopsis, while the late senescence in mutant line of AtPSKR1 supported the idea that PSKR positively regulate the process of leaf senescence. Furthermore, the leaves of the overexpressing lines displayed increased superoxide accumulation compared to mutant lines, along with higher expression of NADPH oxidases (AtRBOHD and AtRBOHF). Importantly, our findings demonstrates that both AtPSKR1 and OsPSKR10 interact with AtRBOHD and AtRBOHF and thereby instigating superoxide accumulation in the leaves through NADPH oxidases."
Authors: Yangdan Li, Yoshiaki Kamiyama, Fuko Minegishi, Yuki Tamura, Kota Yamashita, Sotaro Katagiri, Hinano Takase, Masahiko Otani, Ryo Tojo, Gabrielle E. Rupp, Takamasa Suzuki, Naoto Kawakami, Scott C. Peck and Taishi Umezawa.
The Plant Journal (2024)
Significance Statement: ABA-induced leaf senescence is one of the important processes involved in the translocation of nutrients from one tissue to another under drought stress. We found that group C MAP kinases phosphorylate MBD10 in response to ABA, and the two proteins constitute a regulatory pathway for ABA-induced leaf senescence in Arabidopsis.
Abstract: "Abscisic acid (ABA) is a phytohormone that promotes leaf senescence in response to environmental stress. We previously identified methyl CpG-binding domain 10 (MBD10) as a phosphoprotein that becomes differentially phosphorylated after ABA treatment in Arabidopsis. ABA-induced leaf senescence was delayed in mbd10 knockout plants but accelerated in MBD10-overexpressing plants, suggesting that MBD10 positively regulates ABA-induced leaf senescence. ABA-induced phosphorylation of MBD10 occurs in planta on Thr-89, and our results demonstrated that Thr-89 phosphorylation is essential for MBD10's function in leaf senescence. The in vivo phosphorylation of Thr-89 in MBD10 was significantly downregulated in a quadruple mutant of group C MAPKs (mpk1/2/7/14), and group C MAPKs directly phosphorylated MBD10 in vitro. Furthermore, mpk1/2/7/14 showed a similar phenotype as seen in mbd10 for ABA-induced leaf senescence, suggesting that group C MAPKs are the cognate kinases of MBD10 for Thr-89. Because group C MAPKs have been reported to function downstream of SnRK2s, our results indicate that group C MAPKs and MBD10 constitute a regulatory pathway for ABA-induced leaf senescence."
Authors: Qin Zheng, Zhenning Teng, Jianhua Zhang and Nenghui Ye.
Plants (2024)
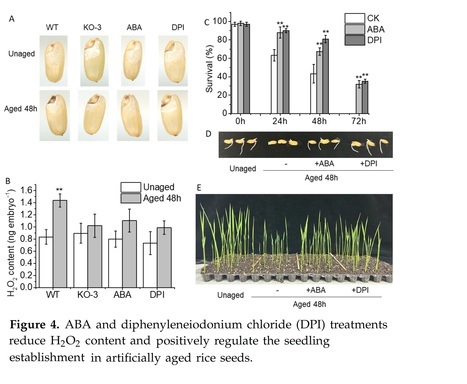
Abstract: "The seed, a critical organ in higher plants, serves as a primary determinant of agricultural productivity, with its quality directly influencing crop yield. Improper storage conditions can diminish seed vigor, adversely affecting seed germination and seedling establishment. Therefore, understanding the seed-aging process and exploring strategies to enhance seed-aging resistance are paramount. In this study, we observed that seed aging during storage leads to a decline in seed vigor and can coincide with the accumulation of hydrogen peroxide (H2O2) in the radicle, resulting in compromised or uneven germination and asynchronous seedling emergence. We identified the abscisic acid (ABA) catabolism gene, abscisic acid 8′-hydroxylase 2 (OsABA8ox2), as significantly induced by aging treatment. Interestingly, transgenic seeds overexpressing OsABA8ox2 exhibited reduced seed vigor, while gene knockout enhanced seed vigor, suggesting its role as a negative regulator. Similarly, seeds pretreated with ABA or diphenyleneiodonium chloride (DPI, an H2O2 inhibitor) showed increased resistance to aging, with more robust early seedling establishment. Both OsABA8ox2 mutant seeds and seeds pretreated with ABA or DPI displayed lower H2O2 content during aging treatment. Overall, our findings indicate that ABA mitigates rice seed aging by reducing H2O2 accumulation in the radicle. This study offers valuable germplasm resources and presents a novel approach to enhancing seed resistance against aging."
Authors: Foziya Altaf, Shazia Parveen, Sumira Farooq, Mohammad Lateef Lone, Aehsan Ul Haq and Inayatullah Tahir
Theoretical and Experimental Plant Physiology (2024)
Abstract: "Due to the already strained and severely challenged agricultural ecosystems of the modern world, predicted changes in life cycle of plants, including leaf senescence are receiving significant attention from stakeholders. The onset, progression and terminal phases of leaf senescence are greatly influenced by plant hormones. The senescence of leaves is accelerated by ethylene, jasmonic acid (JA), salicylic acid (SA), abscisic acid (ABA), brassinosteroids and strigolactones (SLs), whereas it is postponed by cytokinins (CKs), gibberellic acid (GA) and auxins. The crosstalk and signal transduction pathways between these growth regulators have been found to regulate leaf senescence by orchestrating various developmental and environmental factors. Premature leaf senescence lessens the plant’s nutritional capacity and shortens the vegetative production schedule, prompting an early transition from the vegetative to the reproductive stage and diminishing crop potential. As a result, a complete understanding of leaf senescence and finding novel ways to delay it is crucial for agricultural productivity. The ability to manipulate leaf senescence for agricultural enhancement has been made possible by significant advances in physiological and molecular awareness of leaf senescence. Although studies pertaining to leaf senescence have been given steadily more attention, there are still numerous challenges that need to be resolved. In this perspective, this review focuses on current advances in understanding the leaf senescence by molecular and genetic analyses with an emphasis on hormonal regulation of leaf senescence. We also hypothesize future research to better comprehend leaf senescence by employing various current technologies."
Authors: Jingyun Lu, Guifang Zhang, Chao Ma, Yao Li, Chuyan Jiang, Yaru Wang, Bingjie Zhang, Rui Wang, Yuexuan Qiu, Yanxing Ma, Yangchao Jia, Cai-Zhong Jiang, Xiaoming Sun, Nan Ma, Yunhe Jiang and Junping Gao.
The Plant Cell (2024)
One-sentence summary: An ethylene-induced F-box protein, RhSAF, accelerates petal senescence by destabilizing the gibberellic acid receptor RhGID1.
Abstract: "Roses are among the most popular ornamental plants cultivated worldwide for their great economic, symbolic, and cultural importance. Nevertheless, rapid petal senescence markedly reduces rose (Rosa hybrida) flower quality and value. Petal senescence is a developmental process tightly regulated by various phytohormones. Ethylene accelerates petal senescence, while gibberellic acid (GA) delays this process. However, the molecular mechanisms underlying the crosstalk between these phytohormones in the regulation of petal senescence remain largely unclear. Here, we identified SENESCENCE-ASSOCIATED F-BOX (RhSAF), an ethylene-induced F-box protein gene encoding a recognition subunit of the SCF-type E3 ligase. We demonstrated that RhSAF promotes degradation of the GA receptor GIBBERELLIN INSENSITIVE DWARF1 (RhGID1) to accelerate petal senescence. Silencing RhSAF expression delays petal senescence, while suppressing RhGID1 expression accelerates petal senescence. RhSAF physically interacts with RhGID1s and targets them for ubiquitin/26S proteasome-mediated degradation. Accordingly, ethylene-induced RhGID1C degradation and RhDELLA3 accumulation are compromised in RhSAF-RNAi lines. Our results demonstrate that ethylene antagonizes GA activity through RhGID1 degradation mediated by the E3 ligase RhSAF. These findings enhance our understanding of the phytohormone crosstalk regulating petal senescence and provide insights for improving flower longevity."
Authors: Inger B. Holme, Christina R. Ingvardsen, Giuseppe Dionisio, Dagmara Podzimska-Sroka, Kell Kristiansen, Anders Feilberg and Henrik Brinch-Pedersen.
Plant Biotechnology Journal (2024)
Abstract: "Improving tolerance to ethylene-induced early senescence of flowers and fruits is of major economic importance for the ornamental and food industry. Genetic modifications of genes in the ethylene-signalling pathway have frequently resulted in increased tolerance but often with unwanted side effects. Here, we used CRISPR/Cas9 to knockout the function of two CpEil1 genes expressed in flowers of the diploid ornamental plant Campanula portenschlagiana. The ethylene tolerance in flowers of the primary mutants with knockout of only one or all four alleles clearly showed increased tolerance to exogenous ethylene, although lower tolerance was obtained with one compared to four mutated alleles. The allele dosage effect was confirmed in progenies where flowers of plants with zero, one, two, three and four mutated alleles showed increasing ethylene tolerance. Mutation of the Cpeil1 alleles had no significant effect on flower longevity and endogenous flower ethylene level, indicating that CpEil1 is not involved in age-dependent senescence of flowers. The study suggests focus on EIN3/Eils expressed in the organs subjected to early senescence for obtaining tolerance towards exogenous ethylene. Furthermore, the observed allelic dosage effect constitutes a key handle for a gradual regulation of sensitivity towards exogenous ethylene, simultaneously monitoring possibly unwanted side effects."
Authors: Lin Meng, Haipo Yang, Jinli Yang, Yaping Wang, Tiantian Ye, Lin Xiang, Zhulong Chan and Yanping Wang.
Journal of Experimental Botany (2024)
Abstract: "WRKY transcription factors (TFs) play a central role in controlling plant organ senescence. However, it is unclear whether and how WRKY TFs regulate petal senescence in tulip, a widely used ornamental plant. In the present study, we report that TgWRKY75 promoted petal senescence by enhancing abscisic acid (ABA) as well as salicylic acid (SA) synthesis in tulip and in Arabidopsis. The expression level of TgWRKY75 was up-regulated in senescent petals and exogenous ABA or SA treatment induced its expression. The endogenous contents of ABA and SA significantly increased during petal senescence or depended upon TgWRKY75 overexpression. Interestingly, two SA synthesis-related genes TgICS1 and TgPAL1 were identified as direct targets of TgWRKY75 through binding to their promoters. In parallel, TgWRKY75 activated the expression of ABA biosynthesis-related gene TgNCED3 via directly binding to its promoter region. Site mutation of the W-box core motif located on the promoters of TgICS1, TgPAL1 and TgNCED3 eliminated their interactions with TgWRKY75. In summary, this study demonstrated a dual regulation of ABA and SA biosynthesis by TgWRKY75, which unveiled a synergistic process of tulip petal senescence through a feedback regulation between TgWRKY75 and ABA/SA accumulation."
Authors: Yilong Yao, Denghao Xiang, Nai Wu, Yao Wang, Yu Chen, Yang Yuan, Ying Ye, Dan Hu, Chang Zheng, Yu Yan, Qingya Lv, Xiaokai Li, Guoxing Chen, Honghong Hu, Haiyan Xiong, Shaobing Peng and Lizhong Xiong.
Molecular Plant (2023)
Abstract: "Rice ratooning, the fast outgrowth of dormant buds on stubble, is an important cropping practice in rice production. However, the low ratooning ability (RA) of most rice varieties restricts the application of this cost-efficient system, and the genetic basis of RA remains unknown. In this study, we dissected the genetic architecture of RA by a genome-wide association study in a natural rice population. Rice ratooning ability 3 (RRA3), encoding a hitherto not characterized nucleoredoxin involved in reduction of disulfide bonds, was identified as the causal gene of a major locus controlling RA. Overexpression of RRA3 in rice significantly accelerated leaf senescence and reduced RA, whereas knockout of RRA3 significantly delayed leaf senescence and increased RA and ratoon yield. We demonstrated that RRA3 interacts with Oryza sativa histidine kinase 4 (OHK4), a cytokinin receptor, and inhibits the dimerization of OHK4 through disulfide bond reduction. This inhibition ultimately led to decreased cytokinin signaling and reduced RA. In addition, variations in the RRA3 promoter were identified to be associated with RA. Introgression of a superior haplotype with weak expression of RRA3 into the elite rice variety Guichao 2 significantly increased RA and ratoon yield by 23.8%. Collectively, this study not only uncovers an undocumented regulatory mechanism of cytokinin signaling through de-dimerization of a histidine kinase receptor—but also provides an eximious gene with promising value for ratoon rice breeding."
Authors: Koji Nakanishi, Hiroko Fujiki, Koichi Ozaki, Satoko Yanahara, Naoko Takeuchi, Yuji Suzuki, Tamiji Sugiyama, Amane Makino, Taiichiro Ookawa and Tadashi Hirasawa.
Plant and Soil (2023)
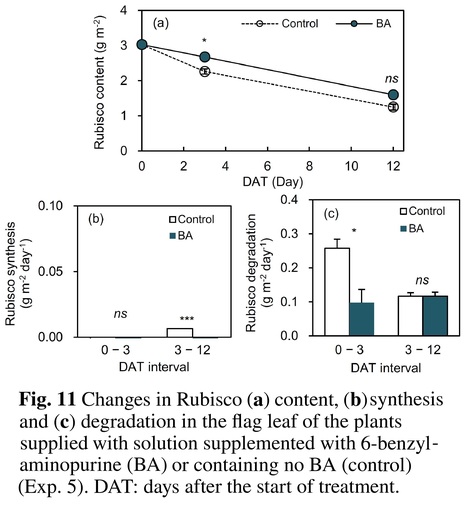
Abstract: "Background and Aims - Leaf senescence is accelerated by soil moisture stress during reproductive growth in rainfed paddy rice under drought and in irrigated paddy rice under intermittent irrigation for saving water or mitigating methane emissions. Leaf senescence decreases leaf photosynthetic rate (An) and grain yield. We aimed to elucidate the mechanisms underlying the An decrease under soil moisture stress. Methods - An, leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), synthesis and degradation of Rubisco and cytokinin flux from roots were compared between plants grown in moisture-deficient soil (DR-plants) and flooded or wet soil (FL- or WE-plants, respectively) during senescence in pot-grown rice (Oryza sativa L.). Results - The decreases in An and Rubisco content were larger in the DR-plants than in the FL-plants. An was closely correlated with Rubisco content during moisture stress treatment. The larger decrease of Rubisco content in the DR-plants was from increased Rubisco degradation rather than decreased synthesis. The amount of cytokinins transported from roots to shoots was smaller in the DR-plants. The application of 6-benzylaminopurine to leaves of the DR-plants suppressed Rubisco degradation. In a wilty mutant with impaired leaf hydraulic conductance, leaf senescence was significantly higher in the DR-plants than in the FL-plants, although leaf water potential of both groups decreased similarly under sunny conditions. Conclusion - The main cause of an An decrease with senescence in rice under soil moisture stress was the decrease of cytokinin flux from roots to shoots and enhanced Rubisco degradation."
|



 Your new post is loading...
Your new post is loading...