Your new post is loading...
 Your new post is loading...
Authors: Jingyi Han (撖静宜), Thomas Welch, Ute Voß, Teva Vernoux, Rahul Bhosale and Anthony Bishopp.
iScience (2024)
Highlights • The first intron of ARF7 is required for expression in the root apical meristem. • It is not required for expression in either lateral roots or the shoot apex. • We find no binding sites in the first intron required for expression in root tips. • We propose that this intron exerts its effects via Intron Mediated Enhancement.
Abstract: "Auxin regulates plant growth and development through the transcription factors of the AUXIN RESPONSE FACTOR (ARF) gene family. ARF7 is one of five activators that bind DNA and elicit downstream transcriptional responses. In roots, ARF7 regulates growth, gravitropism and redundantly with ARF19, lateral root organogenesis. In this study we analyzed ARF7 cis-regulation, using different non-coding sequences of the ARF7 locus to drive GFP. We show that constructs containing the first intron led to increased signal in the root tip. Although bioinformatics analyses predicted several transcription factor binding sites in the first intron, we were unable to significantly alter expression of GFP in the root by mutating these. We instead observed the intronic sequences needed to be present within the transcribed sequences to drive expression in the root meristem. These data support a mechanism by which Intron Mediated Enhancement regulates the tissue specific expression of ARF7 in the root meristem."
Authors: Baris Uzilday, Kaori Takahashi, Akie Kobayashi, Rengin Ozgur Uzilday, Nobuharu Fujii, Hideyuki Takahashi and Ismail Turkan.
Plants (2024)
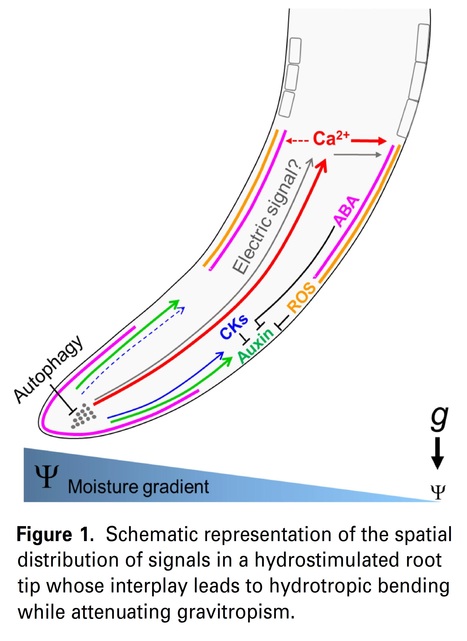
Abstract: "Plant roots exert hydrotropism in response to moisture gradients to avoid drought stress. The regulatory mechanism underlying hydrotropism involves novel regulators such as MIZ1 and GNOM/MIZ2 as well as abscisic acid (ABA), reactive oxygen species (ROS), and Ca2+ signaling. ABA, ROS, and Ca2+ signaling are also involved in plant responses to drought stress. Although the mechanism of moisture gradient perception remains largely unknown, the sensory apparatus has been reported to reside in the root elongation zone rather than in the root cap. In Arabidopsis roots, hydrotropism is mediated by the action of MIZ1 and ABA in the cortex of the elongation zone, the accumulation of ROS at the root curvature, and the variation in the cytosolic Ca2+ concentration in the entire root tip including the root cap and stele of the elongation zone. Moreover, root exposure to moisture gradients has been proposed to cause asymmetric ABA distribution or Ca2+ signaling, leading to the induction of the hydrotropic response. A comprehensive and detailed analysis of hydrotropism regulators and their signaling network in relation to the tissues required for their function is apparently crucial for understanding the mechanisms unique to root hydrotropism. Here, referring to studies on plant responses to drought stress, we summarize the recent findings relating to the role of ABA, ROS, and Ca2+ signaling in hydrotropism, discuss their functional sites and plausible networks, and raise some questions that need to be answered in future studies."
Authors: Juan He, Xiaoyi Li, Qin Yu, Lu Peng, Li Chen, Jiajia Liu, Jianmei Wang, Xufeng Li and Yi Yang.
Plant, Cell & Environment (2024)
Summary statement: The AGC VIII kinase D6 PROTEIN KINASE (D6PK) phosphorylates PIN-FORMED (PIN) auxin efflux carriers, which is essential for auxin transport in plant cells. This study uncovers a new mechanism for D6PK stability through Cytosolic ABA Receptor Kinases-mediated phosphorylation to regulate auxin-controlled Arabidopsis growth and development.
Abstract: "The polar auxin transport is required for proper plant growth and development. D6 PROTEIN KINASE (D6PK) is required for the phosphorylation of PIN-FORMED (PIN) auxin efflux carriers to regulate auxin transport, while the regulation of D6PK stabilization is still poorly understood. Here, we found that Cytosolic ABA Receptor Kinases (CARKs) redundantly interact with D6PK, and the interactions are dependent on CARKs' kinase activities. Similarly, CARK3 also could interact with paralogs of D6PK, including D6PKL1, D6PKL2, and D6PKL3. The genetic analysis shows that D6PK acts the downstream of CARKs to regulate Arabidopsis growth, including hypocotyl, leaf area, vein formation, and the length of silique. Loss-of-function of CARK3 in overexpressing GFP-D6PK plants leads to reduce the level of D6PK protein, thereby rescues plant growth. In addition, the cell-free degradation assays indicate that D6PK is degraded through 26 S proteasome pathway, while the phosphorylation by CARK3 represses this process in cells. In summary, D6PK stabilization by the CARK family is required for auxin-mediated plant growth and development."
Authors: Zachary Aldiss, Hannah Robinson, Yasmine Lam, Richard Dixon, Peter A. Crisp, Ian D. Godwin, Andrew Borrell, Lee Hickey and Karen Massel.
bioRxiv (2024)
Abstract: "Roots provide the critical interface where plants acquire nutrients and water, but our limited understanding of the genetic controls modulating root system architecture (RSA) in crop species constrains opportunities to develop future cultivars with improved root systems. However, there is vast knowledge of root developmental genes in model plant species, which has the potential to accelerate progress in crops with more complex genomes, particularly given that genome editing protocols are now available for most species. PIN-FORMED2 (PIN2) encodes a root specific polar auxin transporter, where its absence resulted in roots being unable to orient themselves using gravity, producing a significantly wider root system. To explore the role of PIN2 in a cereal crop, we used CRISPR/Cas9 editing to knockout of PIN2 in barley (Hordeum vulgare). Like Arabidopsis, the roots of barley pin2 loss-of-function mutants displayed an agravitropic response at seedling growth stages, resulting in a significantly shallower and wider root system at later growth stages. Notably, despite the significant change in RSA, there was no change in shoot architecture or total shoot biomass. We discuss the future challenges and opportunities to harness the PIN2 pathway to optimise RSA in crops for a range of production scenarios without a shoot trade-off."
Authors: Xiuzhen Kong, Yali Xiong, Xiaoyun Song, Samuel Wadey, Suhang Yu, Jinliang Rao, Aneesh Lale, Marco Lombardi, Riccardo Fusi, Rahul Bhosale and Guoqiang Huang.
Plant Physiology (2024)
Abstract: "Root angle is a critical factor in optimising the acquisition of essential resources from different soil depths. The regulation of root angle relies on the auxin-mediated root gravitropism machinery. While the influence of ethylene on auxin levels is known, its specific role in governing root gravitropism and angle remains uncertain, particularly when Arabidopsis (Arabidopsis thaliana) core ethylene signaling mutants show no gravitropic defects. Our research, focusing on rice (Oryza sativa L.) and maize (Zea mays), clearly reveals the involvement of ethylene in root angle regulation in cereal crops through the modulation of auxin biosynthesis and the root gravitropism machinery. We elucidated the molecular components by which ethylene exerts its regulatory effect on auxin biosynthesis to control root gravitropism machinery. The ethylene-insensitive mutants ethylene insensitive2 (osein2) and ethylene insensitive like1 (oseil1), exhibited substantially shallower crown root angle compared to the wild type. Gravitropism assays revealed reduced root gravitropic response in these mutants. Hormone profiling analysis confirmed decreased auxin levels in the root tips of the osein2 mutant, and exogenous auxin (NAA) application rescued root gravitropism in both ethylene-insensitive mutants. Additionally, the auxin-biosynthetic mutant mao hu zi10 (mhz10)/tryptophan aminotransferase2 (ostar2) showed impaired gravitropic response and shallow crown root angle phenotypes. Similarly, maize ethylene-insensitive mutants (zmein2) exhibited defective gravitropism and root angle phenotypes. In conclusion, our study highlights that ethylene controls the auxin-dependent root gravitropism machinery to regulate root angle in rice and maize, revealing a functional divergence in ethylene signaling between Arabidopsis and cereal crops. These findings contribute to a better understanding of root angle regulation and have implications for improving resource acquisition in agricultural systems.
Authors: Ivan Kulich, Julia Schmid, Anastasia Teplova, Linlin Qi and Jiří Friml.
eLife (2024)
Editor's view: This fundamental study addresses the earliest events that enable plant roots to reorient growth in response to gravity. Compelling molecular and cell biological data establish that plasma membrane localization of the LAZY or NEGATIVE GRAVITROPIC RESPONSE OF ROOTS (NGR) protein family is required for rapid and polar redirection of D6 protein kinase, an activator of the PIN3 auxin transporter. This work complements and extends recent publications on the NGR family in gravity sensing (PMID: 37741279 and PMID: 37561884). Collectively these papers advance our understanding of rapid plant gravity sensing and response.
Abstract: "Root gravitropic bending represents a fundamental aspect of terrestrial plant physiology. Gravity is perceived by sedimentation of starch-rich plastids (statoliths) to the bottom of the central root cap cells. Following gravity perception, intercellular auxin transport is redirected downwards leading to an asymmetric auxin accumulation at the lower root side causing inhibition of cell expansion, ultimately resulting in downwards bending. How gravity-induced statoliths repositioning is translated into asymmetric auxin distribution remains unclear despite PIN auxin efflux carriers and the Negative Gravitropic Response of roots (NGR) proteins polarize along statolith sedimentation, thus providing a plausible mechanism for auxin flow redirection. In this study, using a functional NGR1-GFP construct, we visualized the NGR1 localization on the statolith surface and plasma membrane (PM) domains in close proximity to the statoliths, correlating with their movements. We determined that NGR1 binding to these PM domains is indispensable for NGR1 functionality and relies on cysteine acylation and adjacent polybasic regions as well as on lipid and sterol PM composition. Detailed timing of the early events following graviperception suggested that both NGR1 repolarization and initial auxin asymmetry precede the visible PIN3 polarization. This discrepancy motivated us to unveil a rapid, NGR-dependent translocation of PIN-activating AGCVIII kinase D6PK towards lower PMs of gravity-perceiving cells, thus providing an attractive model for rapid redirection of auxin fluxes following gravistimulation."
Authors: Yonatan Wexler, Julian I. Schroeder and Doron Shkolnik.
The Plant Journal (2024)
Significance Statement: Understanding the mechanisms that plant roots utilize to resist the strong tendency to grow downward in order to grow toward a moisture source, holds great potential for unveiling research approaches and technologies to improve crop performance. Herein, we assess the complex interplay between major regulators of root growth, development, and responses to myriad internal and external cues, that function to attenuate downward root growth, thereby enabling the root to reach water.
Abstract: "Plants partly optimize their water recruitment from the growth medium by directing root growth toward a moisture source, a phenomenon termed hydrotropism. The default mechanism of downward growth, termed gravitropism, often functions to counteract hydrotropism when the water-potential gradient deviates from the gravity vector. This review addresses the identity of the root sites in which hydrotropism-regulating factors function to attenuate gravitropism and the interplay between these various factors. In this context, the function of hormones, including auxin, abscisic acid, and cytokinins, as well as secondary messengers, calcium ions, and reactive oxygen species in the conflict between these two opposing tropisms is discussed. We have assembled the available data on the effects of various chemicals and genetic backgrounds on both gravitropism and hydrotropism, to provide an up-to-date perspective on the interactions that dictate the orientation of root tip growth. We specify the relevant open questions for future research. Broadening our understanding of root mechanisms of water recruitment holds great potential for providing advanced approaches and technologies that can improve crop plant performance under less-than-optimal conditions, in light of predicted frequent and prolonged drought periods due to global climate change."
Authors: Jie Le and Yingqiang Long.
Chinese Sci Bulletin (2024)
Abstract: No abstract in English is available.
Authors: Z. Tang, Y. Zhang, Y. Ma, D. Zhao, J. Dong and H. Zhang.
Biologia Plantarum (2024)
Abstract: "Roots are important for plant anchoring, water and nutrient absorption, and other physiological processes. Gravity is a primary determinant of the spatial distribution of plant roots in the soil. Therefore, in-depth understanding of the molecular mechanisms and biochemical networks of root responses to gravity has both theoretical and practical significance in guiding the genetic improvement of plants. Gravitropism, the process through which plants sense the direction of gravity and respond by making the roots grow downward and the stem grow upward, has been widely studied in roots. The perception of gravity and the gravitational growth of roots, key steps in root growth and development, are regulated by auxins and other factors. Here, we review the latest progress in the regulation of root gravitropism by hormone signals and environmental factors from a molecular perspective, and look forward to the direction of future research on root gravitropism."
Author: Asher Elbein
Quanta Magazine (2024).
Subheading: A mutant seedling revealed how plant tissues scatter incoming light, allowing plants to sense its direction and move toward it.
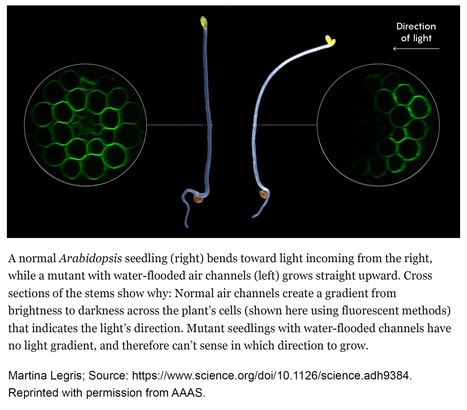
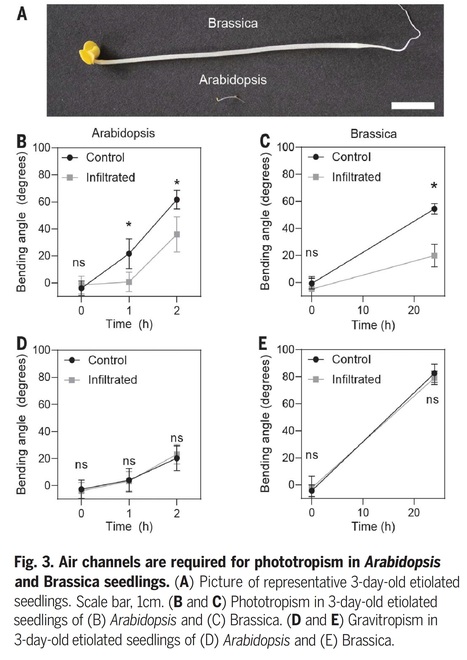
Excerpts: "But in the absence of obvious physical sensing organs like lenses, how do plants work out the precise direction from which light is coming? Now, a team of European researchers has hit upon an answer. In a recent paper published in Science, they report that a roadside weed — Arabidopsis, a favorite of plant geneticists — uses the air spaces between its cells to scatter light, modifying the path of light passing through its tissues. In this way, the air channels create a light gradient that helps seedlings accurately determine where light is coming from. By taking advantage of air channels to scatter light, plants sidestep the need for discrete organs like eyes in favor of a neater trick: the ability in effect to “see” with their whole bodies."
"The researchers deduced that the plant orients itself to light through a mechanism based on the phenomenon of refraction — the tendency of light to change direction as it passes through different media. Because of refraction, Legris explained, light passing through a normal Arabidopsis will scatter under the surface of the stem: Every time it moves through a plant cell, which is mostly water, and then through an air channel, it changes direction. Since some of the light is redirected in the process, the air channels establish a steep light gradient across different cells, which the plant can use to assess the light’s direction and then grow toward it."
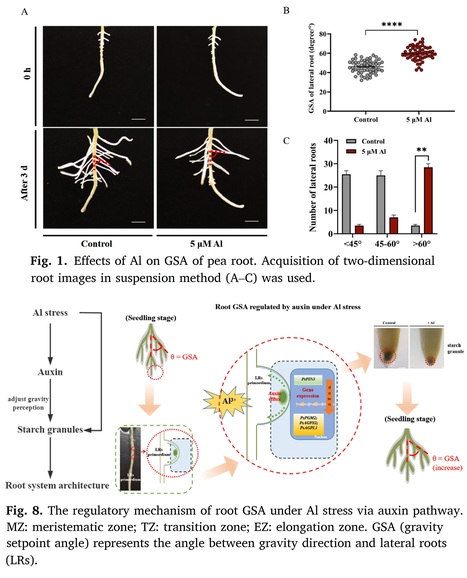
Authors: Hui Wang, Huayang Wang, Houzhou Liu, Tao Wan, Yalin Li, Ketong Zhang, Sergey Shabala, Xuewen Li, Yinglong Chen and Min Yu.
Plant Physiology and Biochemistry (2024)
Highlights • Al stress changed RSA via increasing root GSA of pea. • Exogenous auxin negatively influences the gravitropism of lateral roots by reducing the starch granules in the root tip and changing auxin polar transport. • Al stress changed RSA through the auxin pathway, which is related to the root gravitropic response of plants.
Abstract: "Aluminium (Al) toxicity stands out as a primary cause of crop failure in acidic soils. The root gravity setpoint angle (GSA), one of the important traits of the root system architecture (RSA), plays a pivotal role in enabling plants to adapt to abiotic stress. This study explored the correlation between GSA and Al stress using hydroponic culture with pea (Pisum sativum) plants. The findings revealed that under Al stress, GSA increased in newly developed lateral roots. Notably, this response remained consistent regardless of the treatment duration, extending for at least 3 days during the experiment. Furthermore, exposure to Al led to a reduction in both the size and quantity of starch granules, pivotal components linked to gravity perception. The accumulation of auxin in root transition zone increased. This variation was mirrored in the expression of genes linked to granule formation and auxin efflux, particularly those in the PIN-formed family. This developmental framework suggested a unique role for the root gravitropic response that hinges on starch granules and auxin transport, acting as mediators in the modulation of GSA under Al stress. Exogenous application of indole-3-acetic acid (IAA) and the auxin efflux inhibitor N-1-naphthylphthalamic acid (NPA) had an impact on the root gravitropic response to Al stress. The outcomes indicate that Al stress inhibited polar auxin transport and starch granule formation, the two processes crucial for gravitropism. This impairment led to an elevation in GSA and a reconfiguration of RSA. This study introduces a novel perspective on how plant roots react to Al toxicity, culminating in RSA modification in the context of acidic soil with elevated Al concentrations."
Author: Christopher Whitewoods
Science (2023)
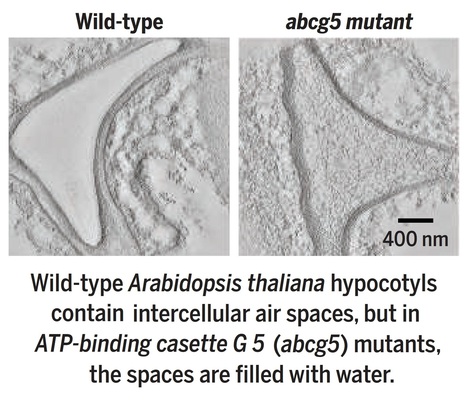
Excerpts: "Decades of work has identified the molecular mechanisms that underlie phototropism (1, 2), but whether plants also physically alter beams of light to enhance their ability to respond was not known. On page 935 of this issue, Nawkar et al. (3) report that plants can use intercellular air spaces to actively modify the path of light and perform accurate phototropism. This finding highlights the importance of physical organ structure in environmental sensing and opens new avenues to understand the role of air spaces in other contexts. Nawkar et al. show that intercellular air spaces in the hypocotyl (embryonic stem) of the model plant Arabidopsis thaliana scatter light and are necessary for phototropism. To do this, they identified a mutant line of the plant with a transparent hypocotyl that does not bend toward light. In this mutant, imaging methods including transmission electron microscopy revealed that the intercellular spaces in the hypocotyl and roots that are normally filled with air are instead full of water (see the images ). These water-filled spaces allowed light to pass through the stem without scattering, making the stem appear transparent. Mutant plants responded normally in response to gravity, which shows that this is a specific response to light rather than a more general defect in environmental sensing or directional growth."
"The authors identified the causative locus in the hypocotyl air space mutant as ATP-BINDING CASETTE G 5 (ABCG5). ABCG5 is a member of a large family of transporters that act on a wide range of substrates (4), although the substrate for ABCG5 is unknown."
Authors: Ning Zhang, Songtao Gui and Yonghong Wang.
Molecular Plant (2023)
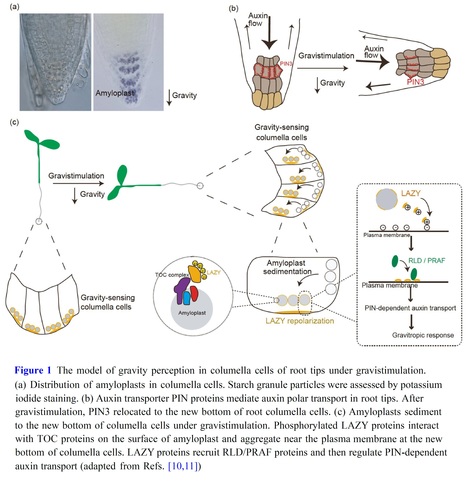
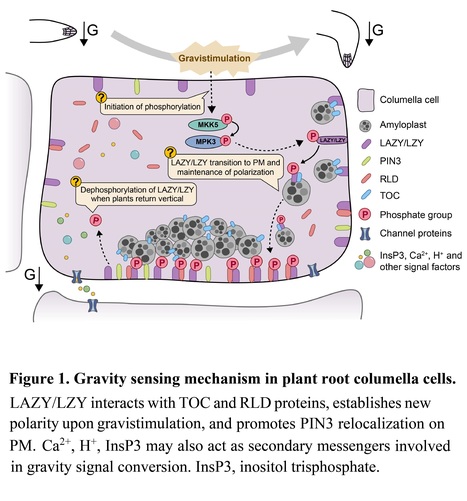
Excerpts: "Recently, two separate research groups have made noteworthy contributions to our understanding of gravity sensing in plants by investigating the role of amyloplast sedimentation in repolarizing LAZY/LZY proteins (Chen et al., 2023; Nishimura et al., 2023). Both studies observed the LAZY/LZY localization on the amyloplast and PM, and translocation of LAZY/LZY from statoliths to the PM in response to gravistimulation."
"Furthermore, Nishimura et al. (2023) discovered that AtLAZY/LZY polar localization can rapidly change upon gravistimulation, resulting in repolarization in the new gravity direction. More importantly, they also explicitly demonstrated the essential role of amyloplast sedimentation in achieving the polarization of AtLAZY/LZY on the PM by manipulating the amyloplasts with the optical tweezer. In this study, authors demonstrated AtLAZY/LZY polarity on the PM is established based on the amyloplast position, achieved through AtLAZY/LZY translocation from amyloplast to the PM."
"Chen et al. (2023) proposed a model for gravity perception in plant roots: Gravistimulation increases the phosphorylation of AtLAZY/LZY mediated by MAPK. This phosphorylation, in turn, strengthens the interaction between AtLAZY/LZY and TOCs on the surface of amyloplasts. Subsequently, amyloplast sedimentation takes AtLAZY/LZY to the new bottom of columella cells. Ultimately, AtLAZY/LZY translocates from amyloplasts to the adjacent PM, establishing a new polarity (Figure 1). These findings represent significant progress in our understanding of gravity sensing mechanisms in plants."
|
Authors: Kei Tsuzuki, Taiki Suzuki, Michio Kuruma, Kotaro Nishiyama, Ken-ichiro Hayashi, Shinya Hagihara and Yoshiya Seto.
bioRxiv (2024)
Abstract: "Root parasitic plants in the Orobancheceae, such as Striga and Orobanche, cause significant damage to crop production. The germination step of these root parasitic plants is induced by host-root-derived strigolactones (SLs). After germination, the radicles elongate toward the host and invade the host root. We have previously discovered that a simple amino acid, tryptophan (Trp), as well as its metabolite, the plant hormone indole-3-acetic acid (IAA), can inhibit radicle elongation of Orobanche minor. These results suggest that auxin plays a crucial role in the radicle elongation step in root parasitic plants. In this report, we used various auxin chemical probes to dissect the auxin function in the radicle growth of O. minor and Striga hermonthica. We found that synthetic auxins inhibited radicle elongation. In addition, auxin receptor antagonist, auxinole, rescued the inhibition of radicle growth by exogenous IAA. Moreover, a polar transport inhibitor of auxin, N-1-naphthylphthalamic acid (NPA), affected radicle tropism. We also proved that exogenously applied Trp is converted into IAA in O. minor seeds, and auxinole partly rescued this radicle elongation. Our data demonstrate a pivotal role of auxin in radicle growth. Thus, manipulation of auxin function in root parasitic plants should offer a useful approach to combat these parasites."
Authors: Ping Yun, Cengiz Kaya and Sergey Shabala.
The Crop Journal (2024)
Abstract: "Salinity stress is a major environmental stress affecting crop productivity, and its negative impact on global food security is only going to increase, due to current climate trends. Salinity tolerance was present in wild crop relatives but significantly weakened during domestication. Regaining it back requires a good understanding of molecular mechanisms and traits involved in control of plant ionic and ROS homeostasis. This review summarizes our current knowledge on the role of major plant hormones (auxin, cytokinins, abscisic acid, salicylic acid, and jasmonate) in plants adaptation to soil salinity. We firstly discuss the role of hormones in controlling root tropisms, root growth and architecture (primary root elongation, meristematic activity, lateral root development, and root hairs formation). Hormone-mediated control of uptake and sequestration of key inorganic ions (sodium, potassium, and calcium) is then discussed followed by regulation of cell redox balance and ROS signaling in salt-stressed roots. Finally, the role of epigenetic alterations such as DNA methylation and histone modifications in control of plant ion and ROS homeostasis and signaling is discussed. This data may help develop novel strategies for breeding and cultivating salt-tolerant crops and improving agricultural productivity in saline regions."
Author: Aida Maric.
Plant Physiology (2024)
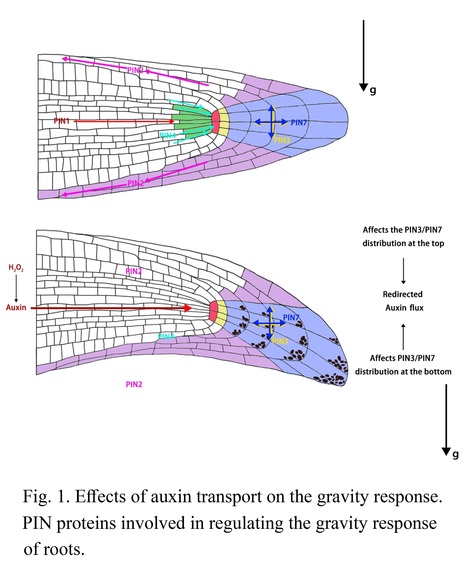
Excerpts: "Recent work by Kong et al. (2024), published in Plant Physiology reports ethylene-auxin hormonal crosstalk as a regulator of root angle in cereal crops. The authors used different root phenotyping methods to compare root response of wild-type and ethylene-insensitive rice and maize mutants. EIL1 and EIN2 are crucial links in the ethylene signaling pathway, conducting ethylene signal from the endoplasmic reticulum to the nucleus to control ethylene-responsive genes. Rice and maize mutants of EIL1 and EIN2 (oseil1, osein2 and zmein2-1) were not able to sense ethylene signals and had impaired ethylene response. Kong et al. (2024) showed that oseil1 and osein2 mutants have a shallower root system with larger root angles (Figure 1A). Additionally, ethylene mutants had an impaired gravitropic response compared to wild-type plants."
"By comparing hormone profiles of ethylene-insensitive mutants with wild-type plants they showed that ethylene-insensitive mutants exhibit lower levels of auxin. Exogenous application of auxin could rescue the root angle phenotype of the ethylene-insensitive mutants (Figure 1B), pinpointing auxin as the intermediate signal in ethylene-mediated root growth angles. Furthermore the authors showed that MHZ10, a member of the auxin biosynthesis pathway, plays a key role in the ethylene-mediated regulation of root angle (Figure 1B)."
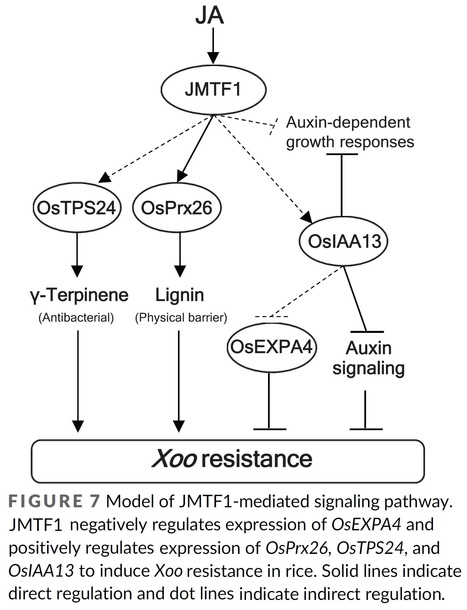
Authors: Yuya Uji, Go Suzuki, Yumi Fujii, Keita Kashihara, Shoko Yamada and Kenji Gomi.
Physiologia Plantarum (2024)
Abstract: "The plant hormone jasmonic acid (JA) is a signalling compound involved in the regulation of cellular defence and development in plants. In this study, we investigated the roles of a JA-responsive MYB transcription factor, JMTF1, in the JA-regulated defence response against rice bacterial blight caused by Xanthomonas oryzae pv. oryzae (Xoo). JMTF1 did not interact with any JASMONATE ZIM-domain (JAZ) proteins. Transgenic rice plants overexpressing JMTF1 showed a JA-hypersensitive phenotype and enhanced resistance against Xoo. JMTF1 upregulated the expression of a peroxidase, OsPrx26, and monoterpene synthase, OsTPS24, which are involved in the biosynthesis of lignin and antibacterial monoterpene, γ-terpinene, respectively. OsPrx26 was mainly expressed in the vascular bundle. Transgenic rice plants overexpressing OsPrx26 showed enhanced resistance against Xoo. In addition to the JA-hypersensitive phenotype, the JMTF1-overexpressing rice plants showed a typical auxin-related phenotype. The leaf divergence and shoot gravitropic responses were defective, and the number of lateral roots decreased significantly in the JMTF1-overexpressing rice plants. JMTF1 downregulated the expression of auxin-responsive genes but upregulated the expression of OsIAA13, a suppressor of auxin signalling. The rice gain-of-function mutant Osiaa13 showed high resistance against Xoo. Transgenic rice plants overexpressing OsEXPA4, a JMTF1-downregulated auxin-responsive gene, showed increased susceptibility to Xoo. JMTF1 is selectively bound to the promoter of OsPrx26 in vivo. These results suggest that JMTF1 positively regulates disease resistance against Xoo by coordinating crosstalk between JA- and auxin-signalling in rice."
Authors: Lulu Zheng, Yongfeng Hu, Tianzhao Yang, Zhen Wang, Daoyuan Wang, Letian Jia, Yuanming Xie, Long Luo, Weicong Qi, Yuanda Lv, Tom Beeckman, Wei Xuan and Yi Han.
Nature Communications (2024)
One-sentence summary: This study reports that the SOMBRERO, a root cap-localized transcription factor, determines root halotropic response to salt stress via spatiotemporally modulating AUX1-dependent auxin redistribution in the root tip.
Abstract: "Plants are capable of altering root growth direction to curtail exposure to a saline environment (termed halotropism). The root cap that surrounds root tip meristematic stem cells plays crucial roles in perceiving and responding to environmental stimuli. However, how the root cap mediates root halotropism remains undetermined. Here, we identified a root cap-localized NAC transcription factor, SOMBRERO (SMB), that is required for root halotropism. Its effect on root halotropism is attributable to the establishment of asymmetric auxin distribution in the lateral root cap (LRC) rather than to the alteration of cellular sodium equilibrium or amyloplast statoliths. Furthermore, SMB is essential for basal expression of the auxin influx carrier gene AUX1 in LRC and for auxin redistribution in a spatiotemporally-regulated manner, thereby leading to directional bending of roots away from higher salinity. Our findings uncover an SMB-AUX1-auxin module linking the role of the root cap to the activation of root halotropism."
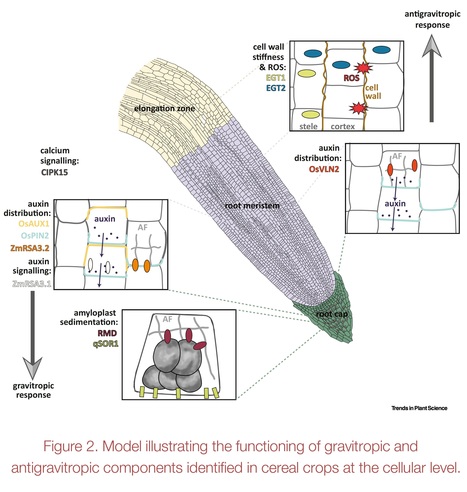
Authors: Gwendolyn K. Kirschner, Frank Hochholdinger, Silvio Salvi, Malcolm J. Bennett, Guoqiang Huang and Rahul A. Bhosale.
Trends in Plant Science (2024)
Highlights: The root angle in cereals determines soil resource capture, stress resilience, and yield, especially in suboptimal conditions. Root angle regulation involves competing gravitropic and antigravitropic offset mechanisms. Understanding the mechanisms underlying root angle regulation in cereals is important due to their complex root system made up of distinct root types, formed at different stages of development. Recent studies in cereals revealed genes regulating the root angle. However, the precise mechanisms determining and maintaining root angle in distinct root types remain unclear. Understanding the molecular mechanisms underlying root angle control is essential for incorporating the root angle trait into breeding programs.
Abstract: "The root angle plays a critical role in efficiently capturing nutrients and water from different soil layers. Steeper root angles enable access to mobile water and nitrogen from deeper soil layers, whereas shallow root angles facilitate the capture of immobile phosphorus from the topsoil. Thus, understanding the genetic regulation of the root angle is crucial for breeding crop varieties that can efficiently capture resources and enhance yield. Moreover, this understanding can contribute to developing varieties that effectively sequester carbon in deeper soil layers, supporting global carbon mitigation efforts. Here we review and consolidate significant recent discoveries regarding the molecular components controlling root angle in cereal crop species and outline the remaining research gaps in this field."
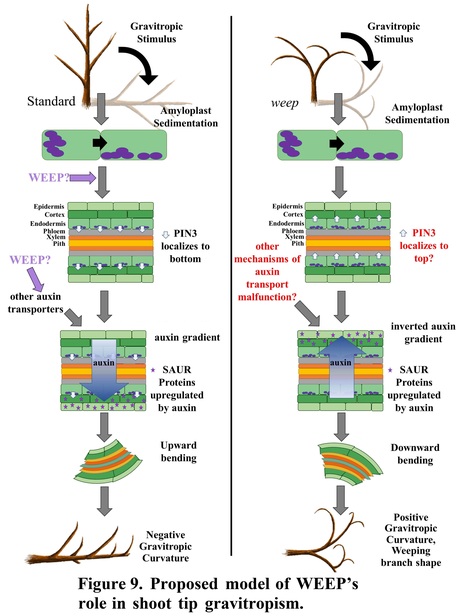
Authors: Andrea R. Kohler, Andrew Scheil, Joseph L. Hill, Jr., Jeffrey R. Allen, Jameel M. Al-Haddad, Charity Z. Goeckeritz, Lucia C. Strader, Frank W. Telewski and Courtney A. Hollender.
Plant Physiology (2024)
One-sentence summary: Polar auxin transport associated with gravitropism and lateral shoot and root orientation requires the highly conserved WEEP protein in peach.
Abstract: "Trees with weeping shoot architectures are valued for their beauty and are a resource for understanding how plants regulate posture control. The peach (Prunus persica) weeping phenotype, which has elliptical downward arching branches, is caused by a homozygous mutation in the WEEP gene. Little is known about the function of WEEP despite its high conservation throughout Plantae. Here, we present the results of anatomical, biochemical, biomechanical, physiological, and molecular experiments that provide insight into WEEP function. Our data suggest that weeping peach trees do not have defects in branch structure. Rather, transcriptomes from the adaxial (upper) and abaxial (lower) sides of standard and weeping branch shoot tips revealed flipped expression patterns for genes associated with early auxin response, tissue patterning, cell elongation, and tension wood development. This suggests that WEEP promotes polar auxin transport toward the lower side during shoot gravitropic response, leading to cell elongation and tension wood development. In addition, weeping peach trees exhibited steeper root systems and faster lateral root gravitropic response. This suggests that WEEP moderates root gravitropism and is essential to establishing the set-point angle of lateral roots from the gravity vector. Additionally, size-exclusion chromatography indicated that WEEP proteins self-oligomerize, like other proteins with sterile alpha motif (SAM) domains. Collectively, our results from weeping peach provide insight into polar auxin transport mechanisms associated with gravitropism and lateral shoot and root orientation."
Authors: Monika Kubalová, Karel Müller, Petre Ivanov Dobrev, Annalisa Rizza, Alexander M. Jones and Matyáš Fendrych.
New Phytologist (2024)
Abstract: "The nuclear TIR1/AFB-Aux/IAA auxin pathway plays a crucial role in regulating plant growth and development. Specifically, the IAA17/AXR3 protein participates in Arabidopsis thaliana root development, response to auxin and gravitropism. However, the mechanism by which AXR3 regulates cell elongation is not fully understood. We combined genetical and cell biological tools with transcriptomics and determination of auxin levels and employed live cell imaging and image analysis to address how the auxin response pathways influence the dynamics of root growth. We revealed that manipulations of the TIR1/AFB-Aux/IAA pathway rapidly modulate root cell elongation. While inducible overexpression of the AXR3-1 transcriptional inhibitor accelerated growth, overexpression of the dominant activator form of ARF5/MONOPTEROS inhibited growth. In parallel, AXR3-1 expression caused loss of auxin sensitivity, leading to transcriptional reprogramming, phytohormone signaling imbalance and increased levels of auxin. Furthermore, we demonstrated that AXR3-1 specifically perturbs nuclear auxin signaling, while the rapid auxin response remains functional. Our results shed light on the interplay between the nuclear and cytoplasmic auxin pathways in roots, revealing their partial independence but also the dominant role of the nuclear auxin pathway during the gravitropic response of Arabidopsis thaliana roots."
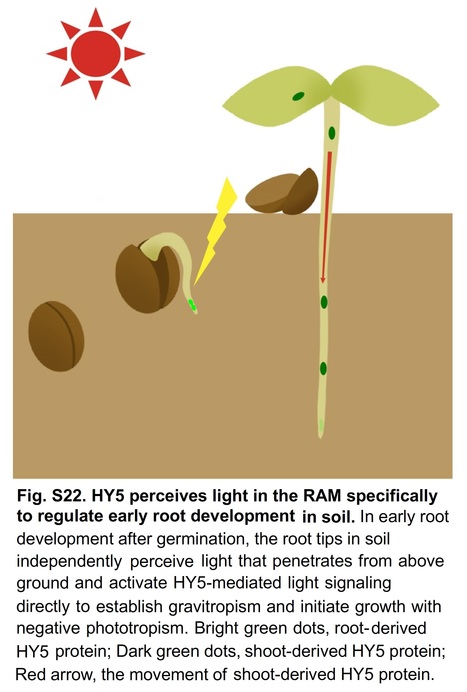
Authors: Jiaojiao Li, Jian Zeng, Zhaoxia Tian and Zhong Zhao.
PNAS (2024)
Significance: Despite growth beneath the soil, the development of roots is tightly controlled by light signaling, the response of which is thought to depend on signal transmission from the shoot. However, whether roots are capable of specifically perceiving light remains unclear. This research shows that the root apical meristem (RAM) perceives light independently from aboveground organs to direct early root development by the light-regulated transcription factor ELONGATED HYPOCOTYL5 (HY5). Root-derived HY5 activates peroxidase expression directly or indirectly by repressing the peroxidase inhibitor to eliminate H2O2 in the RAM to promote root development. Our data suggest that HY5 effects photoreception in the RAM and integrates ROS signaling to direct early root development.
Abstract: "Root development is tightly controlled by light, and the response is thought to depend on signal transmission from the shoot. Here, we show that the root apical meristem perceives light independently from aboveground organs to activate the light-regulated transcription factor ELONGATED HYPOCOTYL5 (HY5). The ROS balance between H2O2 and superoxide anion in the root is disturbed under darkness with increased H2O2. We demonstrate that root-derived HY5 directly activates PER6 expression to eliminate H2O2. Moreover, HY5 directly represses UPBEAT1, a known inhibitor of peroxidases, to release the expression of PERs, partially contributing to the light control of ROS balance in the root. Our results reveal an unexpected ability in roots with specific photoreception and provide a mechanistic framework for the HY5-mediated interaction between light and ROS signaling in early root development."
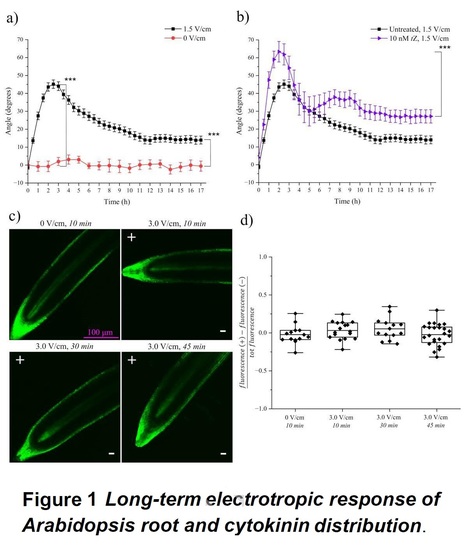
Authors: Maddalena Salvalaio and Giovanni Sena.
Plant Physiology (2024)
Abstract: "Plant roots sense many physical and chemical cues in soil, such as gravity, humidity, light, and chemical gradients, and respond by redirecting their growth towards or away from the source of the stimulus. This process is called tropism. While gravitropism is the tendency to follow the gravitational field downwards, electrotropism is the alignment of growth with external electric fields and the induced ionic currents. Although root tropisms are at the core of their ability to explore large volumes of soil in search of water and nutrients, the molecular and physical mechanisms underlying most of them remain poorly understood. We have previously provided a quantitative characterization of root electrotropism in Arabidopsis (Arabidopsis thaliana) primary roots exposed for 5 hours to weak electric fields, showing that auxin asymmetric distribution is not necessary for root electrotropism but that cytokinin biosynthesis is. Here, we extend that study showing that long-term electrotropism is characterized by a complex behavior. We describe overshoot and habituation as key traits of long-term root electrotropism in Arabidopsis and provide quantitative data about the role of past exposures in the response to electric fields (hysteresis). On the molecular side, we show that cytokinin, although necessary for root electrotropism, is not asymmetrically distributed during the bending. Overall, the data presented here represent a step forward towards a better understanding of the complexity of root behavior and provide a quantitative platform for future studies on the molecular mechanisms of electrotropism."
Authors: Sophie Farkas and Jürgen Kleine-Vehn.
Current Biology (2023)
Summary: Plant gravitropism has fascinated scientists for centuries. A new study provides a major mechanistic update of the so-called starch/statolith hypothesis, revealing how gravity perception is converted into a physiological response.
Excerpts: "The gravitational force serves as a stable reference for plant growth. This information allows plants to orient their shoots and roots vertically, even in the absence of other environmental cues like light. To achieve this, plants must sense gravity and translate this physical stimulus into a physiological response. In a new study, Chen and colleagues1 have now molecularly described the conversion mechanism, allowing plants to relate their growth to gravity."
"Intriguingly, two recent publications1,11 revealed that LZY proteins not only localize to the plasma membrane, but also to the surface of the amyloplasts. Chen and colleagues1 clearly illustrate that LZY proteins sediment together with the amyloplast downwards, where the LZY proteins will subsequently translocate to the plasma membrane of the new bottom side. Accordingly, this intracellular ‘hitchhiking’ mechanism allows the gravity-induced polarization of LZY, ultimately pinpointing the redirection of auxin flow."
"This study provides a molecular explanation for the conversion of a physical signal into a physiological response during gravitropism1. It unveils an intriguing intracellular hitchhiking mechanism that connects amyloplast sedimentation with the asymmetric delivery of LZY proteins to the new lower side of the cell. A central player in this mechanism is the MPK3–MKK5 cascade, which phosphorylates LZY proteins upon gravistimulation, enhancing their interaction with TOC proteins at the amyloplast (Figure 1)."
Authors: Ganesh M. Nawkar, Martina Legris, Anupama Goyal, Emanuel Schmid-Siegert, Jérémy Fleury, Antonio Mucciolo, Damien De Bellis, Martine Trevisan, Andreas Schueler and Christian Fankhauser.
Science (2023)
Editor's view: As seedlings emerge, their embryonic stems unfold and extend as they grow toward light. Phototropin blue light receptors detect the gradient of light, thus allowing the direction of growth to be determined. Nawkar et al. found that mutation of a transporter protein, ABCG5, caused defective seedling phototropism (see the Perspective by Whitewoods). The authors found that air spaces between hypocotyl cells were impaired, preventing scattering of light across the tissue. Intercellular air spaces have been known to aid gas exchange and facilitate buoyancy in aquatic plants. The work here demonstrates an additional functional role for air spaces in setting up a light gradient and also provides insight into how they form.
Abstract: "In plants, light direction is perceived by the phototropin photoreceptors, which trigger directional growth responses known as phototropism. The formation of a phototropin activation gradient across a photosensitive organ initiates this response. However, the optical tissue properties that functionally contribute to phototropism remain unclear. In this work, we show that intercellular air channels limit light transmittance through various organs in several species. Air channels enhance light scattering in Arabidopsis hypocotyls, thereby steepening the light gradient. This is required for an efficient phototropic response in Arabidopsis and Brassica. We identified an embryonically expressed ABC transporter required for the presence of air channels in seedlings and a structure surrounding them. Our work provides insights into intercellular air space development or maintenance and identifies a mechanism of directional light sensing in plants."
|



 Your new post is loading...
Your new post is loading...