Your new post is loading...
 Your new post is loading...
Author: Daniel J. Cosgrove.
Annual Review of Plant Cell and Development (2024)
Abstract: "Expansins comprise an ancient group of cell wall proteins ubiquitous in land plants and their algal ancestors. During cell growth, they facilitate passive yielding of the wall's cellulose networks to turgor-generated tensile stresses, without evidence of enzymatic activity. Expansins are also implicated in fruit softening and other developmental processes and in adaptive responses to environmental stresses and pathogens. The major expansin families in plants include α-expansins (EXPAs), which act on cellulose-cellulose junctions, and β-expansins, which can act on xylans. EXPAs mediate acid growth, which contributes to wall enlargement by auxin and other growth agents. The genomes of diverse microbes, including many plant pathogens, also encode expansins designated expansin-like X. Expansins are proposed to disrupt noncovalent bonding between laterally aligned polysaccharides (notably cellulose), facilitating wall loosening for a variety of biological roles."
Authors: Zhichao Sun, Xinmiao Guo, R.M. Saravana Kumar, Chunying Huang, Yan Xie, Meng Li and Jisheng Li.
Plant Science (2024)
Highlights: • Late stage of mulberry fruit ripening witnessed ethylene accumulation and physiological changes. • Late stage fruit ripening-specific differentially expressed genes and associated metabolic pathways highlighted. • Transcriptome and metabolome data interconnects ethylene signaling and mulberry fruit ripening. • MaERF3 are important for ripening process.
Abstract: "Mulberry (Morus alba L.) is a climacteric and highly perishable fruit. Ethylene has been considered to be an important trigger of fruit ripening process. However, the role of ethylene in the mulberry fruit ripening process remains unclear. In this study, we performed a comprehensive analysis of metabolomic and transcriptomic data of mulberry fruit and the physiological changes accompanying the fruit ripening process. Our study revealed that changes in the accumulation of specific metabolites at different stages of fruit development and ripening were closely correlated to transcriptional changes as well as underlying physiological changes and the development of taste biomolecules. The ripening of mulberry fruits was highly associated with the production of endogenous ethylene, and further application of exogenous ethylene assisted the ripening process. Transcriptomic analysis revealed that differential expression of diverse ripening-related genes was involved in sugar metabolism, anthocyanin biosynthesis, and cell wall modification pathways. Network analysis of transcriptomics and metabolomics data revealed that many transcription factors and ripening-related genes were involved, among which ethylene-responsive transcription factor 3 (MaERF3) plays a crucial role in the ripening process. The role of MaERF3 in ripening was experimentally proven in a transient overexpression assay in apples. Our study indicates that ethylene plays a vital role in modulating mulberry fruit ripening. The results provide a basis for guiding the genetic manipulation of mulberry fruits towards sustainable agricultural practices and improve post-harvest management, potentially enhancing the quality and shelf life of mulberry fruits for sustainable agriculture and forestry."
Authors: James Giovannoni, Yao Chen, Xin Wang, Vincent Colantonio, Tara Fish, Jie Ye, Theodore Thannhauser, Zhibiao Ye, Mingchun Liu, Yongsheng Liu and Zhangjun Fei.
Research Square (2024)
Abstract: "Ripening is crucial for the development of fleshy fruits that release their seeds following consumption by frugivores and are important contributors to human health and nutritional security. Many genetic ripening regulators have been identified, especially in the model system tomato, yet more remain to be discovered and integrated into comprehensive regulatory models. Most tomato ripening genes have been studied in pericarp tissue, though recent evidence indicates that locule tissue is a site of early ripening-gene activities. Here we identified and functionally characterized an Ethylene Response Factor gene, SlERF.D6, by investigating tomato transcriptome data throughout plant development, emphasizing genes elevated in the locule during fruit development and ripening. SlERF.D6loss-of-function mutants resulting from CRISPR/Cas9 gene editing delayed ripening initiation and carotenoid accumulation in both pericarp and locule tissues. Transcriptome analysis of lines altered in SlERF.D6 expression revealed multiple classes of altered genes including ripening regulators, in addition to carotenoid, cell wall and ethylene pathway genes, suggesting comprehensive ripening control. Distinct regulatory patterns in pericarp versus locule tissues were observed indicating tissue-specific activity of this transcription factor. Analysis of SlERF.D6 interaction with target promoters revealed an AP2/ERF transcription factor (SlDEAR2) as a target of SlERF.D6. Furthermore, we show that a third transcription factor gene, SlTCP12, is a target of SlDEAR2, presenting a tri-component module of ripening control."
Authors: Yun Wei, Zhi Liu, Tianxing Lv, Yaxiu Xu, Yajing Wei, Weiting Liu, Li Liu, Aide Wang and Tong Li.
The Plant Cell (2023)
Abstract: "The phytohormone ethylene plays an important role in promoting the softening of climacteric fruits, such as apples (Malus domestica); however, important aspects of the underlying regulatory mechanisms are not well understood. In this study, we identified apple MITOGEN-ACTIVATED PROTEIN KINASE 3 (MdMAPK3) as an important positive regulator of ethylene-induced apple fruit softening during storage. Specifically, we show that MdMAPK3 interacts with and phosphorylates the transcription factor NAM-ATAF1/2-CUC2 72 (MdNAC72), which functions as a transcriptional repressor of the cell wall degradation-related gene POLYGALACTURONASE1 (MdPG1). The increase in MdMAPK3 kinase activity was induced by ethylene, which promoted the phosphorylation of MdNAC72 by MdMAPK3. Additionally, MdPUB24 functions as an E3 ubiquitin ligase to ubiquitinate MdNAC72, resulting in its degradation via the 26S proteasome pathway, which was enhanced by ethylene-induced phosphorylation of MdNAC72 by MdMAPK3. The degradation of MdNAC72 increased the expression of MdPG1, which in turn promoted apple fruit softening. Notably, using variants of MdNAC72 that were mutated at specific phosphorylation sites, we observed that the phosphorylation state of MdNAC72 affected apple fruit softening during storage. This study thus reveals that the ethylene-MdMAPK3-MdNAC72-MdPUB24 module is involved in ethylene-induced apple fruit softening, providing insights into climacteric fruit softening."
Authors: JiaQian Zhou, XiaoYang Zhao, Sen Yang, Cai E. Wu, ZhaoHui Xue and XiaoHong Kou.
Scientia Horticulturae (2023)
Highlights: • SNAC4 and SNAC9 play a significant regulatory role in tomato fruit ripening. • SNAC4/9 induced ethylene and carotenoid biosynthesis, and soluble solid content. • SNAC4 and SNAC9 exhibit different function on ABA biosynthesis and signaling. • SNAC4 and SNAC9 have different regulation modes on fruit softening. • NACs, ABA and ethylene co-regulate tomato fruit ripening.
Abstract: "Previous study suggested that SNAC4 (NM_001279348.2) and SNAC9 (NM_001365397.1) have opposite effects on carotenoid biosynthesis and softening in tomato (Solanum lycopersicum L.) fruit ripening. Here, we use an overexpressing system to explore the regulatory mechanism of SNAC4/9 in tomato fruit ripening and softening. Our results showed that the overexpression of SNAC4 and SNAC9 (OE-SNAC4/9) accelerated fruit ripening, promoted ethylene biosynthesis and carotenoid accumulation, but showed different functions on ABA content and fruit firmness. Genes related to ABA and fruit softening were up-regulated in OE-SNAC4, while down-regulated in OE-SNAC9. The structures of cell wall and peel were also different in these two OE lines. These data verified that SNAC4 and SNAC9 exhibit different regulation modes on fruit softening. Thus, we propose a working model that SNAC4/9 regulates fruit ripening and softening through three layers including cell wall modification-related genes, ethylene and ABA signaling. Our findings provide new insight into NACs involved in regulating fruit ripening."
Authors: Zefeng Zhai, Yuqin Xiao, Yanyan Wang, Yueting Sun, Xiang Peng, Chen Feng, Xiang Zhang, Bingyang Du, Xin Zhou, Chao Wang, Yang Liu and Tianhong Li.
Plant Physiology (2022)
Abstract: "Softening is a key step during fruit ripening that is modulated by the interplay between multiple phytohormones. The antagonistic action of abscisic acid (ABA) and auxin determines the rate of fruit ripening and softening. However, the transcription factors that integrate ABA and auxin signals to regulate fruit softening remain to be determined. In this study, we identified several DNA-binding with One Finger (Dof) transcription factors essential for ABA-promoted fruit softening, based on transcriptome analysis of two sweet cherry (Prunus avium L.) varieties with different fruit firmness. We show that PavDof6 directly binds to the promoters of genes encoding cell wall–modifying enzymes to activate their transcription, while PavDof2/15 directly repress their transcription. Transient overexpression of PavDof6 and PavDof2/15 in sweet cherry fruits resulted in precocious and delayed softening, respectively. In addition, we show that the auxin response factor PavARF8, the expression of whose encoding gene is repressed by ABA, activates PavDof2/15 transcription. Furthermore, PavDof2/6/15 and PavARF8 directly bind to the 9-cis-epoxycarotenoid dioxygenase 1 (PavNCED1) promoter and regulate its expression, forming a feedback mechanism for ABA-mediated fruit softening. These findings unveil the physiological framework of fruit softening and establish a direct functional link between the ABA-PavARF8-PavDofs module and cell wall–modifying genes in mediating fruit softening."
|
Authors: Dongmei Yan and Huilan Yi.
Scientia Horticulturae (2024)
Highlights • Identifying key genes and pathways for postharvest preservation of grapes by RNA-seq. • SO2 prolongs the postharvest shelf life of table grapes by reducing cell wall degradation and enhancing defenses of the skins. • SO2-triggered secondary metabolism and defense responses in grape fruits contributed to postharvest preservation. • SO2-induced inhibition of ethylene signaling pathway helped to delay postharvest softening and aging of grape fruits.
Abstract: "Sulfur dioxide (SO2) is the most frequently used preservative for table grapes, yet the preservation mechanism remains unclear. To ascertain the specific genes and pathways involved in SO2 preservation, RNA-seq technology was employed to characterize the transcriptome profile of grapes during postharvest storage. A total of 22,288 genes were identified, of which 377 genes were differentially expressed (≥ 2-fold change) between SO2 and control groups, mainly enriched in secondary metabolism, plant-pathogen interactions, plant hormone signaling, etc. Numerous genes encoding pathogenesis-related (PR) proteins exhibited higher expression levels in SO2 group, while the activities of disease-resistant enzymes, such as β-1,3-glucanase (PR2) and chitinase (PR3) significantly increased by 28.9 % and 29.3 % (P < 0.05), indicating SO2-induced plant resistance. Exposure to SO2 also enhanced the expression of genes encoding essential enzymes of secondary metabolism, and significantly increased both the activities of critical enzymes for the biosynthesis of secondary metabolites, such as phenylalanine ammonia-lyase (PAL) and 4-coumarate-CoA ligase (4CL), and the contents of secondary metabolites such as total phenol, flavonoid, anthocyanin, and lignin, demonstrating an enhanced chemical and physical barriers in SO2-fumigated grapes. Meanwhile, the genes associated with ethylene signaling and cell wall degradation were down-regulated by SO2 preservative, and some ethylene-responsive elements were identified in the promoter regions of cell wall hydrolase genes, suggesting that SO2-inhibited ethylene signaling might contribute to maintaining cell wall integrity. Altogether, gene expression patterns and cellular physiological processes were altered in grapes exposed to SO2, which helped maintain fruit quality and prolong postharvest life by regulating the defense responses and fruit ageing."
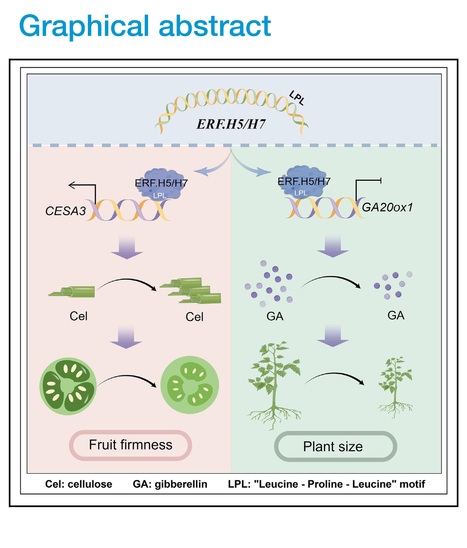
Authors: Yangang Pei, Qihan Xue, Peng Shu, Weijie Xu, Xiaofei Du, Mengbo Wu, Kaidong Liu, Julien Pirrello, Mondher Bouzayen, Yiguo Hong and Mingchun Liu.
Developmental Cell (2024)
Editor's view: Pei et al. demonstrate the role of bifunctional transcription factors SlERF.H5 and SlERF.H7 in activating the cellulose biosynthesis gene SlCESA3 while repressing the gibberellin biosynthesis gene GA20ox1. The study highlights the distinct transcriptional regulatory functions of these ERFs in promoting cell wall formation and inhibiting plant growth.
Highlights • Cellulose is required for cell wall formation and firmness maintenance of fruits • SlERF.H5 and SlERF.H7 act as both transcriptional activators and repressors • The regulatory activity of SlERF.H5 and SlERF.H7 is mediated by a conserved motif
Abstract: "The plant cell wall is a dynamic structure that plays an essential role in development, but the mechanism regulating cell wall formation remains poorly understood. We demonstrate that two transcription factors, SlERF.H5 and SlERF.H7, control cell wall formation and tomato fruit firmness in an additive manner. Knockout of SlERF.H5, SlERF.H7, or both genes decreased cell wall thickness, firmness, and cellulose contents in fruits during early development, especially in double-knockout lines. Overexpressing either gene resulted in thicker cell walls and greater fruit firmness with elevated cellulose levels in fruits but severely dwarf plants with lower gibberellin contents. We further identified that SlERF.H5 and SlERF.H7 activate the cellulose biosynthesis gene SlCESA3 but repress the gibberellin biosynthesis gene GA20ox1. Moreover, we identified a conserved LPL motif in these ERFs responsible for their activities as transcriptional activators and repressors, providing insight into how bifunctional transcription factors modulate distinct developmental processes."
Authors: Ruo-Xi Zhang, Yudi Liu, Xian Zhang, Xiaomei Chen, Juanli Sun, Yun Zhao, Jinyun Zhang, Jia-Long Yao, Liao Liao, Hui Zhou and Yuepeng Han.
New Phytologist (2024)
Abstract: "Although maturity date (MD) is an essential factor affecting fresh fruit marketing and has a pleiotropic effect on fruit taste qualities, the underlying mechanisms remain largely unclear. In this study, we functionally characterized two adjacent NAM-ATAF1/2-CUC2 (NAC) transcription factors (TFs), PpNAC1 and PpNAC5, both of which were associated with fruit MD in peach. PpNAC1 and PpNAC5 were found capable of activating transcription of genes associated with cell elongation, cell wall degradation and ethylene biosynthesis, suggesting their regulatory roles in fruit enlargement and ripening. Furthermore, PpNAC1 and PpNAC5 had pleiotropic effects on fruit taste due to their ability to activate transcription of genes for sugar accumulation and organic acid degradation. Interestingly, both PpNAC1 and PpNAC5 orthologues were found in fruit-producing angiosperms and adjacently arranged in all 91 tested dicots but absent in fruitless gymnosperms, suggesting their important roles in fruit development. Our results provide insight into the regulatory roles of NAC TFs in MD and fruit taste."
Authors: Kenan Jia, Wei Wang, Qing Zhang and Wensuo Jia.
International Journal of Molecular Sciences (2023)
Abstract: "Plant cell walls are essential structures for plant growth and development as well as plant adaptation to environmental stresses. Thus, plants have evolved signaling mechanisms to monitor the changes in the cell wall structure, triggering compensatory changes to sustain cell wall integrity (CWI). CWI signaling can be initiated in response to environmental and developmental signals. However, while environmental stress-associated CWI signaling has been extensively studied and reviewed, less attention has been paid to CWI signaling in relation to plant growth and development under normal conditions. Fleshy fruit development and ripening is a unique process in which dramatic alternations occur in cell wall architecture. Emerging evidence suggests that CWI signaling plays a pivotal role in fruit ripening. In this review, we summarize and discuss the CWI signaling in relation to fruit ripening, which will include cell wall fragment signaling, calcium signaling, and NO signaling, as well as Receptor-Like Protein Kinase (RLKs) signaling with an emphasis on the signaling of FERONIA and THESEUS, two members of RLKs that may act as potential CWI sensors in the modulation of hormonal signal origination and transduction in fruit development and ripening."
Authors: Zhenzhen Peng, Gangshuai Liu, Hongli Li, Yunxiang Wang, Haiyan Gao, Tomislav Jemrić and Daqi Fu.
International Journal of Molecular Sciences (2022)
Abstract: "Fruit softening that occurs during fruit ripening and postharvest storage determines the fruit quality, shelf life and commercial value and makes fruits more attractive for seed dispersal. In addition, over-softening results in fruit eventual decay, render fruit susceptible to invasion by opportunistic pathogens. Many studies have been conducted to reveal how fruit softens and how to control softening. However, softening is a complex and delicate life process, including physiological, biochemical and metabolic changes, which are closely related to each other and are affected by environmental conditions such as temperature, humidity and light. In this review, the current knowledge regarding fruit softening mechanisms is summarized from cell wall metabolism (cell wall structure changes and cell-wall-degrading enzymes), plant hormones (ETH, ABA, IAA and BR et al.), transcription factors (MADS-Box, AP2/ERF, NAC, MYB and BZR) and epigenetics (DNA methylation, histone demethylation and histone acetylation) and a diagram of the regulatory relationship between these factors is provided. It will provide reference for the cultivation of anti-softening fruits."
Authors: Elena Mattus-Araya, Yazmina Stappung, Raúl Herrera and María A. Moya-León.
Journal of Plant Growth Regulation (2023)
Abstract: "Fragaria chiloensis is a native species from Chile and has interesting attributes such as good taste, aroma, and exotic white color. As the maternal relative of commercial strawberry, it is interesting to understand its ripening physiology. Changes in physiological parameters (size, soluble solids, acidity, and firmness) were followed during the development and ripening of F. chiloensis fruit, noticing a fast and intense softening that severely limits its post-harvest shelf life. The content of abscisic acid (ABA) was quantified along its development and displayed an incremental pattern, meanwhile a descendant profile was determined for auxin. To clarify the role of ABA on F. chiloensis softening, detached immature fruits (C2 stage, low ABA levels) were treated with exogenous ABA and changes in firmness were followed during storage at 20 °C. Fruit firmness reduction was accelerated by ABA treatment compared to non-treated fruit, and consistent with that, a clear induction in the accumulation of transcripts of cell wall disassembly genes such as FcPG, FcRGL1, and FcExp5 was observed. In parallel, there was an increment in the transcript level of transcription factors (TFs) such as FcNAC1, FcSEP3, and FcSHP. Good correlations were determined between firmness reduction and the expression of cell wall genes and TFs. Bioinformatic analysis reveals the presence of cis elements responding to ABA in the promoter regions of cell wall disassembly genes and FcNAC1. This indicates that ABA accelerates the set of transcriptional changes leading to softening on this non-climacteric fruit and suggests that these TFs could act as molecular mediators."
|


 Your new post is loading...
Your new post is loading...