Your new post is loading...

|
Scooped by
Juan Lama
|
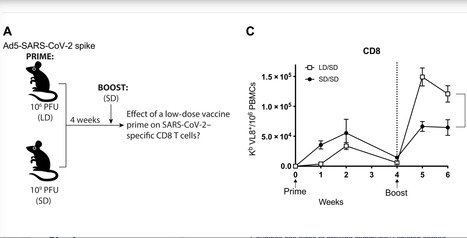
SARS-CoV-2 has caused a global pandemic that has infected more than 250 million people worldwide. Although several vaccine candidates have received emergency use authorization, there is still limited knowledge on how vaccine dosing affects immune responses. We performed mechanistic studies in mice to understand how the priming dose of an adenovirus-based SARS-CoV-2 vaccine affects long-term immunity to SARS-CoV-2. We first primed C57BL/6 mice with an adenovirus serotype 5 vaccine encoding the SARS-CoV-2 spike protein, similar to that used in the CanSino and Sputnik V vaccines. The vaccine prime was administered at either a standard dose or 1000-fold lower dose, followed by a boost with the standard dose 4 weeks later. Initially, the low dose prime induced lower immune responses relative to the standard dose prime. However, the low dose prime elicited immune responses that were qualitatively superior and, upon boosting, exhibited substantially more potent recall and functional capacity. We also report similar effects with a simian immunodeficiency virus (SIV) vaccine. These findings show an unexpected advantage of fractionating vaccine prime doses, warranting a reevaluation of vaccine trial protocols for SARS-CoV-2 and other pathogens. Published in Science Immunology: https://doi.org/10.1126/sciimmunol.abi8635

|
Scooped by
Juan Lama
|
Adenovirus vectors deliver the genetic instructions for SARS-CoV-2 antigens directly into patients' cells, provoking a robust immune response. But will pre-existing immunity from common colds take them down? Six vaccine candidates in clinical trials for COVID-19 employ viruses to deliver genetic cargo that, once inside our cells, instructs them to make SARS-CoV-2 protein. This stimulates an immune response that ideally would protect recipients from future encounters with the actual virus. Three candidates rely on weakened human adenoviruses to deliver the recipe for the spike protein of the pandemic coronavirus, while two use primate adenoviruses and one uses measles virus. Most viral vaccines are based on attenuated or inactivated viruses. An upside of using vectored vaccines is that they are easy and relatively cheap to make. The adenovirus vector, for example, can be grown up in cells and used for various vaccines. Once you make a viral vector, it is the same for all vaccines, says Florian Krammer, a vaccinologist at the Icahn School of Medicine at Mount Sinai. “It is just the genetic information in it that is different,” he explains. Once inside a cell, viral vectors hack into the same molecular system as SARS-CoV-2 and faithfully produce the spike protein in its three dimensions. This resembles a natural infection, which provokes a robust innate immune response, triggering inflammation and mustering B and T cells. But the major downside to the human adenoviruses is that they circulate widely, causing the common cold, and some people harbor antibodies that will target the vaccine, making it ineffective. CanSino reported on its Phase II trial this summer of its COVID-19 vaccine that uses adenovirus serotype 5 (Ad5). The company noted that 266 of the 508 participants given the shot had high pre-existing immunity to the Ad5 vector, and that older participants had a significantly lower immune response to the vaccine, suggesting that the vaccine will not work so well in them. “The problem with adenovirus vectors is that different populations will have different levels of immunity, and different age groups will have different levels of immunity,” says Nikolai Petrovsky, a vaccine researcher at Flinders University in Australia. Also, with age, a person accumulates immunity to more serotypes. “Being older is associated with more chance to acquire Ad5 immunity, so those vaccines will be an issue [with elderly people],” Krammer explains. Moreover, immunity against adenoviruses lasts for many years. “A lot of people have immunity to Ad5 and that impacts on how well the vaccine works,” says Krammer. In the US, around 40 percent of people have neutralizing antibodies to Ad5. As part of her work on an HIV vaccine, Hildegund Ertl of the Wistar Institute in Philadelphia previously collected serum in Africa to gauge resistance levels to this and other serotypes. She found a high prevalence of Ad5 antibodies in sub-Saharan Africa and some West African countries—80 to 90 percent. A different group in 2012 reported that for children in northeast China, around one-quarter had moderate levels and 9 percent had high levels of Ad5 antibodies. “I don’t think anyone has done an extensive enough study to do a world map [of seroprevalence],” notes Ertl. J&J’s Janssen is using a rarer adenovirus subtype, Ad26, in its COVID-19 vaccine, reporting in July that it protects macaques against SARS-CoV-2 and in September that it protects against severe clinical disease in hamsters. Ad26 neutralizing antibodies are uncommon in Europe and the US, with perhaps 10–20 percent of people harboring antibodies. They are more common elsewhere. “In sub-Saharan Africa, the rates are ranging from eighty to ninety percent,” says Ertl. Also critical is the level of antibodies in individuals, notes Dan Barouch, a vaccinologist at Beth Israel Deaconess Medical Center and Harvard Medical School. For instance, there was no neutralizing of Ad26-based HIV and Ebola vaccines in more than 80,000 people in sub-Saharan Africa, he says. “Ad26 vaccine responses do not appear to be suppressed by the baseline Ad26 antibodies found in these populations,” because the titres are low, Barouch writes in an email to The Scientist. Barouch has long experience with Ad26-based vaccines and collaborates with J&J on their COVID-19 vaccine. The Russian Sputnik V vaccine, approved despite no published data or Phase 3 trial results, starts with a shot of Ad26 vector followed by a booster with Ad5, both of which carry the gene for the spike protein of SARS-CoV-2. This circumvents a downside of viral vector vaccines, specifically, once you give the first shot, subsequent injections will be less efficacious because of antibodies against the vector. Ertl says she has no idea of the proportion of the Russian population with Ad26 or Ad5 antibodies, and there seems to be little or no published data from countries that have expressed interested in this virus, such as Venezuela and the Philippines...

|
Scooped by
Juan Lama
|
High-profile COVID-19 vaccines developed in Russia and China share a potential shortcoming: They are based on a common cold virus that many people have been exposed to, potentially limiting their effectiveness, some experts say. CanSino Biologics’ vaccine, approved for military use in China, is a modified form of adenovirus type 5, or Ad5. The company is in talks to get emergency approval in several countries before completing large-scale trials, the Wall Street Journal reported last week. A vaccine developed by Moscow’s Gamaleya Institute, approved in Russia earlier this month despite limited testing, is based on Ad5 and a second less common adenovirus. “The Ad5 concerns me just because a lot of people have immunity,” said Anna Durbin, a vaccine researcher at Johns Hopkins University. “I’m not sure what their strategy is … maybe it won’t have 70 percent efficacy. It might have 40 percent efficacy, and that’s better than nothing, until something else comes along.” Vaccines are seen as essential to ending the pandemic that has claimed over 845,000 lives worldwide. Gamaleya has said its two-virus approach will address Ad5 immunity issues. Both developers have years of experience and approved Ebola vaccines based on Ad5. Neither CanSino nor Gamaleya responded to requests for comment. Researchers have experimented with Ad5-based vaccines against a variety of infections for decades, but none are widely used. They employ harmless viruses as “vectors” to ferry genes from the target virus — in this case the novel coronavirus — into human cells, prompting an immune response to fight the actual virus. But many people already have antibodies against Ad5, which could cause the immune system to attack the vector instead of responding to the coronavirus, making these vaccines less effective. Several researchers have chosen alternative adenoviruses or delivery mechanisms. Oxford University and AstraZeneca based their COVID-19 vaccine on a chimpanzee adenovirus, avoiding the Ad5 issue. Johnson & Johnson’s candidate uses Ad26, a comparatively rare strain. Dr. Zhou Xing, from Canada’s McMaster University, worked with CanSino on its first Ad5-based vaccine, for tuberculosis, in 2011. His team is developing an inhaled Ad5 COVID-19 vaccine, theorizing it could circumvent pre-existing immunity issues. “The Oxford vaccine candidate has quite an advantage” over the injected CanSino vaccine, he said. Xing also worries that high doses of the Ad5 vector in the CanSino vaccine could induce fever, fueling vaccine skepticism. “I think they will get good immunity in people that don’t have antibodies to the vaccine, but a lot of people do,” said Dr. Hildegund Ertl, director of the Wistar Institute Vaccine Center in Philadelphia. In China and the United States, about 40 percent of people have high levels of antibodies from prior Ad5 exposure. In Africa, it could be has high as 80 percent, experts said. Some scientists also worry an Ad5-based vaccine could increase chances of contracting HIV. In a 2004 trial of a Merck & Co Ad5-based HIV vaccine, people with pre-existing immunity became more, not less, susceptible to the virus that causes AIDS. Researchers, including top U.S. infectious diseases expert Dr. Anthony Fauci, in a 2015 paper, said the side effect was likely unique to HIV vaccines. But they cautioned that HIV incidence should be monitored during and after trials of all Ad5-based vaccines in at-risk populations. Gamaleya’s vaccine will be administered in two doses: The first based on Ad26, similar to J&J’s candidate, and the second on Ad5. Alexander Gintsburg, Gamaleya’s director, has said the two-vector approach addresses the immunity issue. Ertl said it might work well enough in individuals who have been exposed to one of the two adenoviruses.

|
Scooped by
Juan Lama
|
Immune responses that are associated with a decreased risk of infection are called correlates of protection (CoP). Clinical trials that measure vaccine-induced antibody and cell-mediated immune responses help to define CoP to HIV, which are necessary to assess the efficacy of a promising HIV vaccine candidate.A pilot study led by senior authors Georgia Tomaras and Peter Gilbert and first authors Scott Neidich, Youyi Fong and Shuying Li of the NIAID-funded HIV Vaccine Trials Network (HVTN) demonstrated that an increase in three antibody-mediated immune responses (antibody-mediated Fcƴ receptor [FcƴR] recruitment, antibody-dependent cellular phagocytosis [ADCP], and anti-Env IgG3) correlated with a decrease in HIV transmission. To date, only the RV144/Thai trial has demonstrated moderate efficacy for an experimental HIV vaccine. RV144 identified two primary immune correlates—IgG antibodies to the variable regions of HIV Env correlated with decreased risk of HIV transmission, and plasma IgA to Env correlated with decreased vaccine efficacy. As only one HIV vaccine trial has shown efficacy thus far, the RV144 immune correlates should be corroborated in an independent study to confirm the CoP are true surrogate markers for protection from HIV acquisition. To date, only the RV144/Thai trial has demonstrated moderate efficacy for an experimental HIV vaccine. RV144 identified two primary immune correlates—IgG antibodies to the variable regions of HIV Env correlated with decreased risk of HIV transmission, and plasma IgA to Env correlated with decreased vaccine efficacy. As only one HIV vaccine trial has shown efficacy thus far, the RV144 immune correlates should be corroborated in an independent study to confirm the CoP are true surrogate markers for protection from HIV acquisition. To test whether the antibody functions were independent correlates of HIV risk, ADCP and antibody-mediated FcƴR recruitment (antibody-mediated immune responses) were statistically assessed and the polyfunctionality score for CD4+ and CD8+ T cells (cellular-mediated immune responses) were measured. The authors found that both the antibody- and cellular-mediated immune responses significantly correlated with the decreased transmission of HIV. Individual Env IgG3 (subclass of antibody) measurements significantly correlated with reduced risk of HIV transmission. Env IgA responses modulated antibody Fc effector functions supporting the previously identified RV144 IgA CoR. To best predict the transmission of HIV, this study demonstrated that measuring both antibody and cellular mediated immune responses are necessary. The antibody and cellular mediated immune responses at four weeks after the final vaccination (month 7) were determined in a blinded case control study of 125 HVTN 505 participants who did not acquire HIV and 25 participants who acquired HIV. Previous studies confirmed that vaccine-induced CD8+ T cells impact viral load; however it is not known if antibody Fc effector functions can play a protective role after HIV infection in humans. The researchers examined the relationship between the strength of antibody Fc effector functions with viral load (VL) set point in those vaccinees who acquired HIV. The pilot study confirmed that antibody effector function significantly correlated with lower VL set point in those vaccinees who acquired HIV. Notably, of the antibody responses, ADCP and IgG3 were among the strongest correlates associated with the decrease in HIV transmission; however, Fc?RIIa engagement was the only correlate of decreased VL in infected vaccinees. ADCP, IgG3 and FcR engagement are emerging hypotheses that can be tested as a CoP upon completion of the HVTN 702, HVTN 705, and HVTN 706 large-scale HIV vaccine efficacy trials underway globally. Published in J. Clinical Investigation (October 7, 2019): https://doi.org/10.1172/JCI126391
|

|
Scooped by
Juan Lama
|
We are writing to express concern about the use of a recombinant adenovirus type-5 (Ad5) vector for a COVID-19 phase 1 vaccine study,1 and subsequent advanced trials. Over a decade ago, we completed the Step and Phambili phase 2b studies that evaluate an Ad5 vectored HIV-1 vaccine administered in three immunisations for efficacy against HIV-1 acquisition. Both international studies found an increased risk of HIV-1 acquisition among vaccinated men. The Step trial found that men who were Ad5 seropositive and uncircumcised on entry into the trial were at elevated risk of HIV-1 acquisition during the first 18 months of follow-up. The hazard ratios were particularly high among men who were uncircumcised and Ad5 seropositive, and who reported unprotected insertive anal sex with a partner who was HIV-1 seropositive or had unknown serostatus at baseline, suggesting the potential for increased risk of penile acquisition of HIV-1. Importantly for considering the potential use of Ad5 vectors for COVID-19 infection, a similar increased risk of HIV infection was also observed in heterosexual men who enrolled in the Phambili study. This effect appeared to persist over time. Both studies involved an Ad5 construct that did not have the HIV-1 envelope. In another HIV study, done only in men who were Ad5 seronegative and circumcised, a DNA prime followed by an Ad5 vector were used, in which both constructs contained the HIV-1 envelope. No increased risk of HIV infection was noted. A consensus conference about Ad5 vectors held in 2013 and sponsored by the National Institutes of Health indicated the most probable explanation for these differences related to the potential counterbalancing effects of envelope immune responses in mitigating the effects of the Ad5 vector on HIV-1 acquisition. The conclusion of this consensus conference warned that non-HIV vaccine trials that used similar vectors in areas of high HIV prevalence could lead to an increased risk of HIV-1 acquisition in the vaccinated population. The increased risk of HIV-1 acquisition appeared to be limited to men; a similar increase in risk was not seen in women in the Phambili trial... Published in The Lancet (October 19, 2020): https://doi.org/10.1016/S0140-6736(20)32156-5

|
Scooped by
Juan Lama
|
Russia's "Sputnik-V" COVID-19 vaccine produced an antibody response in all participants in early-stage trials, according to results published on Friday by The Lancet medical journal that were hailed by Moscow as an answer to its critics. The results of the two trials, conducted in June-July this year and involving 76 participants, showed 100% of participants developing antibodies to the new coronavirus and no serious side effects, The Lancet said. Russia licensed the two-shot jab for domestic use in August, the first country to do so and before any data had been published or a large-scale trial begun. “The two 42-day trials – including 38 healthy adults each – did not find any serious adverse effects among participants, and confirmed that the vaccine candidates elicit an antibody response,” The Lancet said. “Large, long-term trials including a placebo comparison, and further monitoring are needed to establish the long-term safety and effectiveness of the vaccine for preventing COVID-19 infection,” it said. The vaccine is named Sputnik-V in homage to the world’s first satellite, launched by the Soviet Union. Some Western experts have warned against its use until all internationally approved testing and regulatory steps have been taken. But with the results now published for the first time in an international peer-reviewed journal, and with a 40,000-strong later-stage trial launched last week, a senior Russian official said Moscow had faced down its critics abroad. “With this (publication) we answer all of the questions of the West that were diligently asked over the past three weeks, frankly with the clear goal of tarnishing the Russian vaccine,” said Kirill Dmitriev, the head of the Russian Direct Investment Fund (RDIF), Russia’s sovereign wealth fund, which has backed the vaccine. “All of the boxes are checked,” he told Reuters. “Now... we will start asking questions of some of the Western vaccines.” Commenting on the results of the early-stage trials, lead author Dr Naor Bar-Zeev of the International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, USA said the studies were “encouraging but small”. Bar-Zeev, who was not involved in the study, said “clinical efficacy for any COVID-19 vaccine has not yet been shown.” “The report is a case of ‘so far, so good’,” Brendan Wren, Professor of Microbial Pathogenesis at London’s School of Hygiene and Tropical Medicine said. Original study in The Lancet (Sept. 4, 2020): https://doi.org/10.1016/S0140-6736(20)31866-3

|
Scooped by
Juan Lama
|
A Covid-19 vaccine candidate being developed by a Chinese drug maker appeared to induce an immune response in subjects, but also showed some concerning although not unexpected results. Data on the vaccine, made by CanSino Biologics, were published Friday in the Lancet, the first time Phase 1 trial data from any Covid-19 vaccine have been published in a scientific journal. The results are likely to be closely examined, particularly in Canada, which recently announced it would test the vaccine and produce it there if results of the early studies were positive. The study found that one dose of the vaccine, tested at three different levels, appeared to induce a good immune response in some subjects. But about half of the volunteers — people who already had immunity to the backbone of the vaccine — had a dampened immune response. The vaccine is what’s known as a viral vector vaccine; it uses a live but weakened human cold virus, adenovirus 5, onto which genetic material of the SARS-CoV-2 coronavirus has been fused. The Ad5 virus is effectively a delivery system that teaches the immune system to recognize the coronavirus. But many people have had previous infections with adenovirus 5, raising concerns that the immune system would focus on the Ad5 parts of the vaccine rather than the SARS-Cov-2 part. Many research groups that work on viral-vectored vaccines stopped using Ad5 because of concerns about preexisting immunity, which can run to 70% or higher in some populations. “This is definitely one of the concerns about using vectored vaccines for which people might already have pre-existing immunity,” said Michael Mina, an infectious diseases epidemiologist at Harvard’s T.H. Chan School of Public Health. “If you already have seen a virus or have some pre-existing immunity to it … you run the risk of having your immune response get skewed and picking up primarily the thing you’re already immune to or that you’ve already seen and not focusing so much on the new aspect, which in this case would be the coronavirus proteins that were placed onto the adenovirus vector,” he said. In the study, Chinese scientists reported that while people who had high levels of preexisting immunity to Ad5 responded to the vaccine, the rise in antibodies to the SARS-Cov-2 virus was less robust than among those in the study who had low or no preexisting antibodies to Ad5. They also showed antibodies to the adenovirus itself soared among people who had prior immunity, suggesting their systems views the vaccination as a boost of their Ad5 immunity..... Studiy Published in The Lancet (May 22, 2020): https://doi.org/10.1016/S0140-6736(20)31208-3
|



 Your new post is loading...
Your new post is loading...