Your new post is loading...

|
Scooped by
Juan Lama
|
Dormant HIV actively produces RNA and proteins during anti-retroviral therapy. Patients with HIV usually carry a reservoir of HIV-infected cells that can re-emerge if anti-retroviral therapy stops. These reservoirs have long been thought to be dormant, but two independent groups of researchers report that a subset of these cells spontaneously produce HIV RNA and proteins that may impact patients’ HIV-specific immune responses. Their findings are published in the journal Cell Host & Microbe in the articles titled: “Spontaneous HIV expression during suppressive ART is associated with the magnitude and function of HIV-specific CD4+ and CD8+ T cells” and “An active HIV reservoir during ART is associated with maintenance of HIV-specific CD8+ T cell magnitude and short-lived differentiation status.” “It’s a deceptively dormant virus,” said immunovirologist Daniel Kaufmann, MD, of the University Hospital of Lausanne, the University of Lausanne, and l’Université de Montréal, and who is senior author on one of the papers. “Even in people who are treated, HIV continues to have some activity, and it continues to interact with the immune system. We have to understand if these ongoing interactions have clinically relevant consequences.” Previous studies have shown that when “dormant” HIV reservoir cells are reactivated in the lab, they produce viral RNA and proteins, but it wasn’t clear whether this phenomenon was happening inside the bodies of people with HIV. “We wanted to understand if this phenomenon is real and, if it is, what parts of the virus are being expressed and do they have an impact on the immune system,” explained Kaufmann. The researchers took blood samples from 18 people with HIV who had all been on antiretrovirals for more than three years. Then, they used a lab method called RNA flow cytometry to sort CD4+ or “helper” T cells based on whether they were infected with HIV and, then further, whether they were actively producing HIV RNA or proteins. The researchers also characterized the T cells by role—e.g., whether they were the type of T helper cell that combats intracellular viruses or the type that combats extracellular bacteria—to determine whether any subtypes of CD4+ T cells were more likely to host HIV reservoirs. “Our technique allows us to look at the individual cells to see if they contain the virus and which parts of the virus they are expressing,” said Mathieu Dubé, an immunovirologist at l’Université de Montréal and first author on the paper led by Kaufmann. “For each patient, we could estimate how many of these cells are still active, and we could also look for associations between viral characteristics and cell characteristics.” The researchers observed that 14 of the 18 patients had HIV reservoirs that spontaneously produced viral RNA. For seven of the 18 patients, the viral reservoirs also produced viral proteins including p24, a component of HIV’s shell. “Most of the virus that remains in the body is defective or junk virus that cannot really multiply, but we found these defective viruses can still produce virus RNA and sometimes proteins,” said Kaufmann. “Our data suggest that the RNA and proteins produced by these viral reservoirs could be drivers of inflammation,” said Kaufmann. “This could be important because a subset of the people who are successfully treated with anti-retroviral therapy for HIV still have negative consequences of living with the infection—for example, accelerated cardiac disease, frailty, and premature osteoporosis.” The second paper, led by immunologist Lydie Trautmann, PhD, at the Vaccine and Gene Therapy Institute of Oregon Health and Science University, also reported that a subset of CD4+ T cells spontaneously express viral RNA during anti-retroviral therapy. “Our study suggests that residual immune dysfunction driven by the active HIV reservoir on anti-retroviral therapies could contribute to the lack of viral control after analytical treatment interruption by preventing the differentiation of functional stem-like self-renewing HIV-specific CD8+ T cells that can mount efficient rapid recall responses,” the authors wrote. “Therefore, HIV remission strategies will likely need to target transcriptionally active proviruses producing viral proteins during anti-retroviral therapies to harness HIV-specific CD8+ T cells to control rebounding of the virus after therapy cessation.” Research cited published in Cell and Host Microbe (September 2023): https://doi.org/10.1016/j.chom.2023.08.006

|
Scooped by
Juan Lama
|
Participants in the trial must be between the ages of 18 and 50 and not diagnosed with HIV. Moderna is set to start human trials for its experimental mRNA HIV vaccine as early as Thursday, the first time such a trial has ever been conducted. The big picture: "There's a pressing need for new ways to prevent infection from viruses like HIV and influenza that conventional vaccines have struggled to address and to treat rare genetic diseases and cancers that kill millions each year," Axios' Alison Snyder writes. "Vaccines and therapies based on messenger RNA (mRNA) hold promise as a solution." Details: To participate in the trial, participants have to be between the ages of 18 and 50 and not diagnosed with HIV. Moderna says it is looking for 56 participants. What they're saying: "Even as we have shown that our mRNA-based vaccine can prevent Covid-19, this has encouraged us to pursue more-ambitious development programs within our prophylactic vaccines modality," said Moderna CEO Stéphane Bancel in January, when the company announced the vaccine, per Clinical Trials Arena.

|
Scooped by
Juan Lama
|
The "mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine," the CDC wrote in updated guidance. The Centers for Disease Control and Prevention (CDC) issued new COVID-19 vaccine guidance, which indicates that those with preexisting medical conditions should receive the vaccine. "Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19," the CDC wrote in updated guidance on December 26, adding that "mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine." The two different vaccines that are currently being administered to Americans use mRNA technology, which tells the body to create antibodies to combat the virus, instead of using a weakened version of the virus. The two vaccines include one developed by Pfizer and German biotech company, BioNTech and the other developed by Moderna. The CDC's COVID-19 vaccine update provides guidance for those with several different preexisting medical conditions including weakened immune systems, autoimmune conditions, Guillain-Barre syndrome and Bell's palsy. As the CDC notes, people with HIV and weakened immune systems "due to other illnesses or medication might be at increased risk for severe COVID-19." The CDC states that these people should receive a COVID-19 vaccine but notes that "information about the safety of mRNA COVID-19 vaccines" for this group is not yet available. "People living with HIV were included in clinical trials, though safety data specific to this group are not yet available at this time," the CDC said. The CDC provides similar guidance for those with autoimmune conditions, stating that this group should receive a COVID-19 vaccine but adds that "they should be aware that no data are currently available on the safety of mRNA COVID-19 vaccines for them." According to the CDC, to date, there have been no reported cases of Guillain-Barre syndrome after a person received a COVID-19 vaccine during clinical trials and people who previously had GBS should receive the vaccine. On December 17, the Food and Drug Administration reported cases of Bell's palsy in people who received the Pfizer and Moderna vaccines during clinical trials. Bell's palsy is a temporary facial paralysis, but in the CDC's updated guidance, it states that the FDA "does not consider these [cases] to be above the rate expected in the general population." "They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell's palsy may receive an mRNA COVID-19 vaccine," the CDC stated. The CDC's updated guidance also states that even after receiving a COVID-19 vaccine, people should continue to follow the current guidance to combat the virus. The current guidelines include wearing a protective face mask while in public, following social distancing measures, washing hands, and following quarantine guidance after being exposed to the virus. The updated guidance comes as the CDC and many other health authorities have said that those with preexisting medical conditions are at a heightened risk of developing serious cases of the novel virus. Newsweek reached out to the CDC for comment but did not receive a response in time for publication. The updated guidance comes as the CDC and many other health authorities have said that those with preexisting medical conditions are at a heightened risk of developing serious cases of the novel virus. Newsweek reached out to the CDC for comment but did not receive a response in time for publication. Updated CDC guidelines (Dec. 29, 2020): https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/underlying-conditions.html

|
Scooped by
Juan Lama
|
HPTN 083 is the first study to compare the efficacy of CAB LA to daily oral TDF/FTC for HIV PrEP. HPTN 083 enrolled 4,570 cisgender men who have sex with men (MSM) and transgender women (TGW) who have sex with men at 43 sites in Argentina, Brazil, Peru, United States, South Africa, Thailand and Vietnam. For many people, taking a daily pill can be challenging. The development of safe and effective long acting alternative agents for HIV PrEP would increase HIV prevention choices and help those who find taking a daily pill challenging. Some people also may find periodic injections to be a more discreet form of HIV PrEP than daily pills and may prefer CAB LA for that reason. Participants were assigned randomly (by chance) to either the CAB or oral TDF/FTC group. Neither the participants nor the study team knows who was in which group. Participants in each group received both injections and oral tablets – each participant received one active drug and one placebo (no active drug) in order to maintain the blinded nature of the study. Participants were randomized to one of two study arms and included three steps: Step 1 consisted of 5 weeks of daily oral CAB and a TDF/FTC placebo or 5 weeks of daily oral TDF/FTC and an oral CAB placebo; Step 2 consisted of 148 weeks of intramuscular CAB LA 600 mg every 8 weeks plus daily oral TDF/FTC placebo or 148 weeks of daily oral TDF/FTC plus an intramuscular CAB LA placebo every 8 weeks ; and Step 3 consisted of an open-label daily oral TDF/FTC for 48 weeks after participants completed Step 2. On May 14, 2020 a Data and Safety Monitoring Board (DSMB) reviewed HPTN 083 study data and recommended that the blinded part of the study be stopped early for successfully meeting its specified objectives. The study results showed that CAB LA, administered every eight weeks, provided high efficacy compared to TDF/FTC. A total of 50 incident HIV infections occurred in HPTN 083, with 38 incident HIV infections in the TDF/FTC arm (incidence rate 1.21%) and 12 incident HIV infections in the CAB arm (incidence rate 0.38%): in other words, approximately three times the number of incident HIV infections were in the TDF/FTC arm than in the CAB arm. The study sponsor, the U.S. National Institute of Allergy and Infectious Diseases (NIAID), approved the decision to stop the blinded part of the study.
|

|
Scooped by
Juan Lama
|
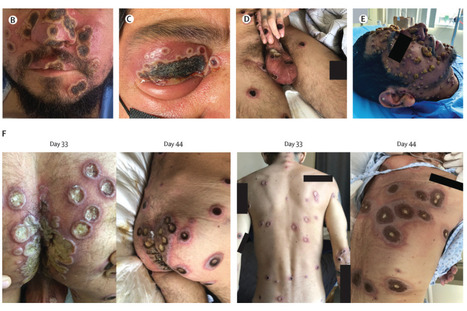
Background People living with HIV have accounted for 38–50% of those affected in the 2022 multicountry mpox outbreak. Most reported cases were in people who had high CD4 cell counts and similar outcomes to those without HIV. Emerging data suggest worse clinical outcomes and higher mortality in people with more advanced HIV. We describe the clinical characteristics and outcomes of mpox in a cohort of people with HIV and low CD4 cell counts (CD4 <350 cells per mm3). Methods A network of clinicians from 19 countries provided data of confirmed mpox cases between May 11, 2022, and Jan 18, 2023, in people with HIV infection. Contributing centres completed deidentified structured case report sheets to include variables of interest relevant to people living with HIV and to capture more severe outcomes. We restricted this series to include only adults older than 18 years living with HIV and with a CD4 cell count of less than 350 cells per mm3 or, in settings where a CD4 count was not always routinely available, an HIV infection clinically classified as US Centers for Disease Control and Prevention stage C. We describe their clinical presentation, complications, and causes of death. Analyses were descriptive. Findings We included data of 382 cases: 367 cisgender men, four cisgender women, and ten transgender women. The median age of individuals included was 35 (IQR 30–43) years. At mpox diagnosis, 349 (91%) individuals were known to be living with HIV; 228 (65%) of 349 adherent to antiretroviral therapy (ART); 32 (8%) of 382 had a concurrent opportunistic illness. The median CD4 cell count was 211 (IQR 117–291) cells per mm3, with 85 (22%) individuals with CD4 cell counts of less than 100 cells per mm3 and 94 (25%) with 100–200 cells per mm3. Overall, 193 (51%) of 382 had undetectable viral load. Severe complications were more common in people with a CD4 cell count of less than 100 cells per mm3 than in those with more than 300 cells per mm3, including necrotising skin lesions (54% vs 7%), lung involvement (29% vs 0%) occasionally with nodules, and secondary infections and sepsis (44% vs 9%). Overall, 107 (28%) of 382 were hospitalised, of whom 27 (25%) died. All deaths occurred in people with CD4 counts of less than 200 cells per mm3. Among people with CD4 counts of less than 200 cells per mm3, more deaths occurred in those with high HIV viral load. An immune reconstitution inflammatory syndrome to mpox was suspected in 21 (25%) of 85 people initiated or re-initiated on ART, of whom 12 (57%) of 21 died. 62 (16%) of 382 received tecovirimat and seven (2%) received cidofovir or brincidofovir. Three individuals had laboratory confirmation of tecovirimat resistance. Interpretation A severe necrotising form of mpox in the context of advanced immunosuppression appears to behave like an AIDS-defining condition, with a high prevalence of fulminant dermatological and systemic manifestations and death. Published in The Lancet (feb. 21, 2023):

|
Scooped by
Juan Lama
|
Investigators believe that an effective HIV vaccine would need to produce broadly neutralizing antibodies that would work on different strains of the virus. A phase 1 trial of a novel approach for HIV vaccination successfully stimulated production of rare immune cells needed to start the process of generating antibodies against the virus. Notably, the targeted response was detected in 97% of participants who received the vaccine, according to a press release. This study demonstrates proof of principle for a new vaccine concept for HIV, a concept that could be applied to other pathogens, as well,” said William Schief, PhD, whose laboratory developed the vaccine, in a press release. “With our many collaborators on the study team, we showed that vaccines can be designed to stimulate rare immune cells with specific properties, and this targeted stimulation can be very efficient in humans. We believe this approach will be key to making an HIV vaccine and possibly important for making vaccines against other pathogens.”
HIV affects more than 38 million people globally and is known to be one of the most difficult viruses to target with a vaccine. One of the major challenges is because the virus constantly evolves into different strains to evade the immune system. Investigators have worked for decades to create a vaccine that would stimulate the immune system to create rare but powerful antibodies called broadly neutralizing antibodies (bnAbs). According to the press release, bnAbs are specialized blood proteins that could attach to HIV spikes and disable them via important yet difficult-to-access regions that don’t vary much from strain to strain. “We and others postulated many years ago that in order to induce bnAbs, you must start the process by triggering the right B cells—cells that have special properties giving them potential to develop into bnAb-secreting cells,” Schief explained in the press release. “In this trial, the targeted cells were only about 1 in a million of all naïve B cells. To get the right antibody response, we first need to prime the right B cells. The data from this trial affirms the ability of the vaccine immunogen to do this.”
The strategy of targeting naïve B cells with specific properties is called germline-targeting, because these young B cells display germline genes. Investigators believe the approach could also be applied to vaccines for other challenging pathogens, such as influenza, dengue, Zika, hepatitis C virus, and malaria. The clinical trial, IAVI G001, enrolled 48 healthy adult volunteers who received either a placebo or 2 doses of the vaccine compound along with an adjuvant developed by GSK. According to the investigators, the novel design, the clinical trial, and the molecular B cell analyses provide a roadmap for further progress in an HIV vaccine. “This is a tremendous achievement for vaccine science as a whole,” said Dennis Burton, PhD, scientific director of the IAVI Neutralizing Antibody Center and director of the NIH Consortium for HIV/AIDS Vaccine Development, in a press release. “This clinical trial has shown that we can drive immune responses in predictable ways to make new and better vaccines, and not just for HIV. We believe this type of vaccine engineering can be applied more broadly, bringing about a new day in vaccinology.”
February 3, 2021: https://www.scripps.edu/news-and-events/press-room/2021/20210203-hiv-vaccine.html.

|
Scooped by
Juan Lama
|
The UNAIDS estimates that 38 million people currently live with human immunodeficiency virus (HIV) infection. Combination antiretroviral treatment has had great success in saving lives but is also associated with numerous medical and public health challenges. Vaccination remains the best and most cost-effective option for controlling HIV infection across the world. Professor Tomáš Hanke jointly from the University of Oxford, UK, and Kumamoto University, Japan, designs vaccines and coordinates clinical programs testing the most advanced vaccine candidates developed by his team in the UK, Europe, U.S. and Africa. Ending AIDS with vaccination Human immunodeficiency virus (HIV) type 1 represents 95% of all HIV infections worldwide and is responsible for the global HIV pandemic. If untreated, HIV-infected patients develop acquired immunodeficiency syndrome—better known as AIDS—that manifests as a progressive failure of their immune system. As a result, patients eventually succumb to opportunistic infections. Combination antiretroviral treatment (cART) has transformed the lives of people living with HIV, and dramatically decreased AIDS-related mortality and onward transmission of HIV. Unfortunately, the provision of cART to everybody who needs it faces many obstacles particularly in low- and middle-income countries. The cost, complexity of the treatment, necessity of regular monitoring of patients, threat of drug resistance, side effects, social stigma and the use of cART to prevent HIV infections (or pre-exposure prophylaxis), which further stretches the cART supply, make cART a suboptimal therapeutic and an unlikely stand-alone tool to end the HIV epidemic. Therefore, an effective vaccine for both prevention and cure of HIV is urgently needed. Professor Tomáš Hanke and his team at the Jenner Institute at the University of Oxford, UK, together with their collaborators at the Joint Research Center for Human Retrovirus Infection, Kumamoto University, Japan, are studying T cell responses to HIV to inform vaccine development. In addition, Professor Hanke oversees Experimental Medicine trials of his leading T-cell vaccine candidates in healthy and HIV-positive people at several global sites and collaborates with prestigious universities and organizations such as the International AIDS Vaccine Initiative, IrsiCaixa AIDS Research Institute-HIVACAT in Spain, Imperial College London, the Kenya AIDS Vaccine Initiative-Institute for Clinical Research and National Institute of Allergy and Infectious Diseases. He also co-ordinates the "Globally Relevant AIDS Vaccine Europe-Africa Trials Partnership" consortium, acronymed GREAT, which builds research capacity and tests vaccine candidates in Eastern and Southern Africa, and is one of the principal investigators of the European AIDS Vaccine Initiative 2020. Rational iterative development Most of today's HIV vaccine research focuses on antibody-mediated immunity, which neutralizes cell-free viruses and typically involves exposing people to the outer HIV spike. However, to achieve HIV control, antibodies may need to be complemented by T-cell responses, the focus of Professor Hanke's research. There is no doubt that T cells contribute in an important way to anti-HIV immunity, whereby CD8 T cells known as "killer cells" directly kill virus-infected cells, the virus factories, and CD4 T cells or 'helper cells' assist and co-ordinate antibody and T-cell induction. "The trick is to induce not just any, but protective killer T cells that can slow or stop HIV," explains Professor Hanke. The first clinically tested vaccine that Professor Hanke and his colleagues developed was called HIVA. It was derived from an African clade A strain of HIV and was tested in over a dozen clinical trials. Following the field's full appreciation of the HIV's enormous ability to change, Professor Hanke improved his approach by focusing vaccine-elicited T cells on the functionally conserved regions of HIV, which are common to most HIV strains and essential for virus survival. If successful, such a vaccine could be deployed universally in all global regions. The prototype conserved immunogen was called HIVconsv (to emphasize conserved in addition to consensus sequences) and assembled highly conserved HIV regions into a chimeric protein alternating the global major HIV strains. This vaccine showed encouraging results in initial small clinical trials and informed the design of the second-generation conserved vaccines called HIVconsvX. Notable HIVconsvX improvements include the use of bioinformatics to redefine conserved regions and increase the vaccine match to the global HIV variants by using a so-called "mosaic" design. The HIVconsvX vaccines entered clinical evaluation in 2019 with further trials in the pipeline...

|
Scooped by
Juan Lama
|
Scientists at the University of Arizona were able to extract from the tissue a nearly complete genetic sequence of an HIV virus. For more than 50 years, the DNA remained hidden in a lymph node that had been snipped out of a 38-year-old man in what is now the Democratic Republic of the Congo. That nub of tissue, the size of a nail on a pinky finger, had been sealed up in a protective block of paraffin. The sample that was examined dates from 1966. The sequence extracted from it is older by a decade than the previous oldest full-length sequence. It provides a snapshot of what the virus looked like when it was circulating undetected in central Africa 15 years before a cluster of strange infections among gay men in the United States led to recognition of a new disease that was eventually called AIDS. Worobey, whose lab has repeatedly performed virologic archeology on old tissue and blood samples, said the paper has not yet been submitted to a scientific journal for publication. As such, it has not been through the peer review process where independent scientists kick its tires, so to speak. Genetic codes of viruses that infected people in earlier days of the AIDS epidemic can be used by scientists to try to date when the HIV virus moved from primates into people. By studying differences in the viral sequences, scientists estimate how long it has been since the known sequences could have diverged from a common source. It doesn’t tell them when the event happened, but it can suggest that it had to have been prior to a particular time, Worobey said, adding that the new data suggest the jump likely did not happen in the 1920s. Findings were pre-printed in the bioRxiv website: https://www.biorxiv.org/content/10.1101/687863v1
|



 Your new post is loading...
Your new post is loading...