Your new post is loading...

|
Scooped by
Juan Lama
|
We analyzed the kinetics and durability of the humoral responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and vaccination using >8,000 longitudinal samples collected over a three-year period (April 2020 to April 2023) in the New York City metropolitan area. Upon primary immunization, participants with pre-existing immunity mounted higher antibody responses faster and achieved higher steady-state levels compared to naive individuals. Antibody durability was characterized by two phases: an initial rapid decay, followed by a phase of stabilization with very slow decay resulting in an individual spike binding antibody steady state. Booster vaccination equalized the differences in antibody levels between participants with and without hybrid immunity, but the antibody titers reached decreased with each successive antigen exposure. Break-through infections increased antibody titers to similar levels as an additional vaccine dose in naive individuals. Our study provides strong evidence for the fact that SARS-CoV-2 antibody responses are long lasting, with an initial waning phase followed by a stabilization phase. Preprint available at medRxiv (August 28, 2023): https://doi.org/10.1101/2023.08.26.23294679

|
Scooped by
Juan Lama
|
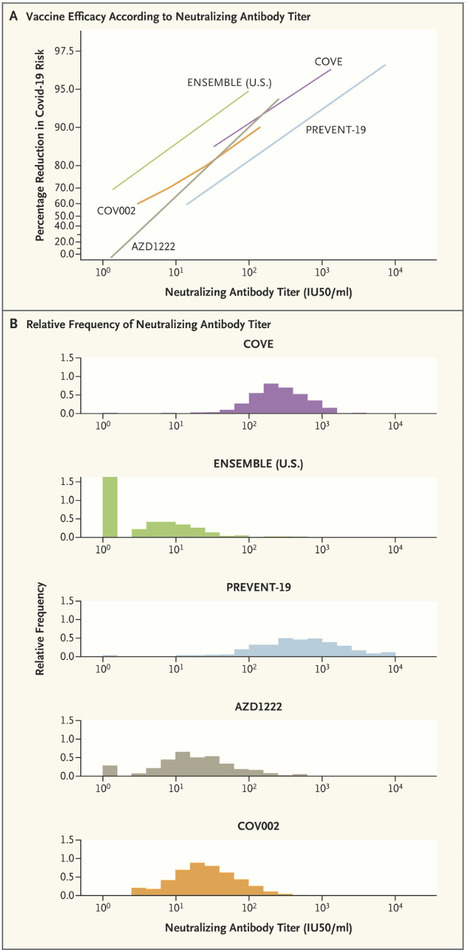
The rapid identification of a correlate of protection (CoP) for Covid-19 vaccines — on the basis of several harmonized randomized phase 3 trials using common validated assays — constitutes an important success in vaccinology. A CoP is an immune marker that can be used to reliably predict a vaccine’s level of efficacy in preventing a clinically relevant outcome. The level of this marker is measured shortly (2 to 4 weeks) after completion of the vaccination regimen and provides an actionable basis for decisions such as regulatory approval of an efficacious vaccine for a new population that was not included in the pivotal randomized phase 3 trials, or approval of a refined version of a vaccine that was previously shown to be efficacious. Once established, a CoP can be used as the primary end point for provisional or full approval of a vaccine for a specific use, if a clinical immunobridging study confirms that high enough levels of the CoP are achieved. For example, the Food and Drug Administration (FDA) extended approval of the mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech) Covid vaccines from older to younger age groups on the basis of a comparison of neutralizing antibody titers. Moreover, FDA guidance and a European Medicines Agency declaration from the International Coalition of Medicines Regulatory Authorities recommended that approval of new vaccine strains and booster doses be based on clinical immunobridging studies showing noninferiority or superiority with respect to a CoP end point. Other applications of a CoP include ensuring vaccine consistency from lot to lot, supporting recommendations for coadministration with other vaccines, and determination of appropriate expiration dates. Confusion about CoPs is understandable, given the myriad complicated issues involved in identifying them and the fact that different uses for CoPs require different validation measures. Evidence that a marker is a CoP is generally derived from five main sources: natural history studies that correlate infection-induced immune responses with outcomes; vaccine-challenge studies in animals or humans; studies that experimentally manipulate the immune marker to directly assess mechanistic causation (e.g., by administering various vaccine doses or using passive antibody transfer); efficacy trials that quantify the relationship between vaccine efficacy and the level of the immune marker in individual vaccine recipients; and meta-analyses of series of efficacy trials that correlate vaccine efficacy with the mean immune-marker level. Strong evidence has been generated from all five of these sources for both serum anti-spike IgG concentration and anti–SARS-CoV-2 neutralizing antibody titer as CoPs for vaccines against symptomatic Covid-19; for brevity, we focus here on the neutralizing antibody titer. Meta-analyses have established high correlations between the standardized mean titer and vaccine efficacy, and the neutralizing antibody titer has consistently been shown to be a mechanistic CoP in challenge studies in nonhuman primates. The U.S. government’s COVID-19 Vaccine Correlates of Protection Program assessed CoPs in phase 3 trials of four vaccines: COVE for mRNA-1273, ENSEMBLE for Ad26.COV2.S, PREVENT-19 for NVX-CoV2373, AZD1222 (United States/Chile/Peru) for ChAdOx1 nCoV-19, and COV002 (United Kingdom) also for ChAdOx1 nCoV-19. Vaccine efficacy always markedly increased with the titer. Published in NEJM (Dec. 10, 2022): https://doi.org/10.1056/NEJMp2211314

|
Scooped by
Juan Lama
|
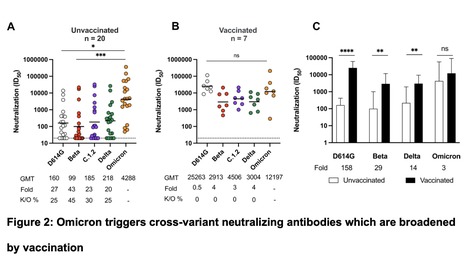
The SARS-CoV-2 Omicron variant largely escapes neutralizing antibodies elicited by vaccines or infection. However, whether Omicron triggers humoral responses that are cross-reactive to other variants of concern (VOCs) remains largely unknown. We use plasma from 20 unvaccinated and seven vaccinated individuals infected during the Omicron wave in South Africa to test binding, antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and neutralization against VOCs. In unvaccinated individuals, Fc effector function and binding antibodies target Omicron and other VOCs at comparable levels. However, Omicron-triggered neutralization is not extensively cross-reactive to VOCs, with 20 to 43-fold reductions in titer. In contrast, vaccination followed by breakthrough Omicron infection improved cross-neutralization of VOCs, with titers exceeding 1:2,900. This has important implications for the vulnerability of unvaccinated Omicron-infected individuals to reinfection by circulating and emerging VOCs. Further, while Omicron-based immunogens may be adequate boosters, they are unlikely to be superior to existing vaccines for priming in SARS-CoV-2 naïve individuals. Preprint available (Feb. 14, 2022): https://www.medrxiv.org/content/10.1101/2022.02.10.22270789v1

|
Scooped by
Juan Lama
|
The mRNA-based Moderna vaccine spurs an immune response that persists for at least six months. The two-dose vaccine made by Moderna in Cambridge, Massachusetts, has been shown to be 94% effective at preventing COVID-19. To learn whether the vaccine provides lasting protection, Mehul Suthar at Emory University School of Medicine in Decatur, Georgia, and his colleagues studied antibodies collected from 33 people who received the vaccine during an early phase of testing (N. Doria-Rose et al. N. Engl. J. Med. https://doi.org/f5c6; 2021). Three types of test showed that participants still had antibodies against the coronavirus six months after receiving their second dose of the vaccine. For example, antibodies from all participants, including those in the oldest age group, could inhibit a modified version of SARS-CoV-2 in the laboratory. The authors are now studying whether antibodies elicited by the vaccine last for more than six months. Findings Published in N. Engl. J. Medicine (April 6, 2021): https://doi.org/10.1056/NEJMc2103916

|
Scooped by
Juan Lama
|
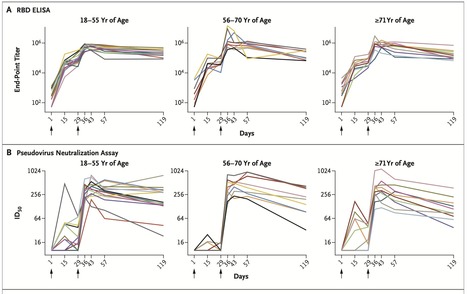
We recently reported the results of a phase 1 trial of a messenger RNA vaccine, mRNA-1273, to prevent infection with SARS-CoV-2; those interim results covered a period of 57 days after the first vaccination. Here, we describe immunogenicity data 119 days after the first vaccination (90 days after the second vaccination) in 34 healthy adult participants in the same trial who received two injections of vaccine at a dose of 100 μg. The injections were received 28 days apart. The recipients were stratified according to age (18 to 55 years, 56 to 70 years, or ≥71 years), and the assays used have been described previously. At the 100-μg dose, mRNA-1273 produced high levels of binding and neutralizing antibodies that declined slightly over time, as expected, but they remained elevated in all participants 3 months after the booster vaccination. Binding antibody responses to the spike receptor–binding domain were assessed by enzyme-linked immunosorbent assay. At the day 119 time point, the geometric mean titer (GMT) was 235,228 (95% confidence interval [CI], 177,236 to 312,195) in participants 18 to 55 years of age, 151,761 (95% CI, 88,571 to 260,033) in those 56 to 70 years of age, and 157,946 (95% CI, 94,345 to 264,420) in those 71 years of age or older Published in NEJM (Dec. 3, 2020): https://doi.org/10.1056/NEJMc2032195

|
Scooped by
Juan Lama
|
Antibodies that people make to fight the new coronavirus last for at least four months after diagnosis and do not fade quickly as some earlier reports suggested, scientists have found. Tuesday’s report, from tests on more than 30,000 people in Iceland, is the most extensive work yet on the immune system’s response to the virus over time, and is good news for efforts to develop vaccines. If a vaccine can spur production of long-lasting antibodies as natural infection seems to do, it gives hope that “immunity to this unpredictable and highly contagious virus may not be fleeting,” scientists from Harvard University and the U.S. National Institutes of Health wrote in a commentary published with the study in the New England Journal of Medicine. One of the big mysteries of the pandemic is whether having had the coronavirus helps protect against future infection, and for how long. Some smaller studies previously suggested that antibodies may disappear quickly and that some people with few or no symptoms may not make many at all. The new study was done by Reykjavik-based deCODE Genetics, a subsidiary of the U.S. biotech company Amgen, with several hospitals, universities and health officials in Iceland. The country tested 15% of its population since late February, when its first COVID-19 cases were detected, giving a solid base for comparisons. Scientists used two different types of coronavirus testing: the kind from nose swabs or other samples that detects bits of the virus, indicating infection, and tests that measure antibodies in the blood, which can show whether someone was infected now or in the past. Blood samples were analyzed from 30,576 people using various methods, and someone was counted as a case if at least two of the antibody tests were positive. These included a range of people, from those without symptoms to people hospitalized with signs of COVID-19. In a subgroup who tested positive, further testing found that antibodies rose for two months after their infection initially was diagnosed and then plateaued and remained stable for four months. Previous studies suggesting antibodies faded quickly may have been just looking at the first wave of antibodies the immune system makes in response to infection; those studies mostly looked 28 days after diagnosis. A second wave of antibodies forms after a month or two into infection, and this seems more stable and long-lasting, the researchers report. The results don’t necessarily mean that all countries’ populations will be the same, or that every person has this sort of response. Other scientists recently documented at least two cases where people seem to have been reinfected with the coronavirus months after their first bout. The new study does not establish how much or which type of antibody confers immunity or protection — that remains unknown. The study also found: — Testing through the bits-of-virus method that’s commonly done in community settings missed nearly half of people who were found to have had the virus by blood antibody testing. That means the blood tests are far more reliable and better for tracking spread of the disease in a region and for guiding decisions and returning to work or school, researchers say. — Nearly a third of infections were in people who reported no symptoms. — Nearly 1% of Iceland’s population was infected in this first wave of the pandemic, meaning the other 99% are still vulnerable to the virus. — The infection fatality rate was 0.3%. That’s about three times the fatality rate of seasonal flu and in keeping with some other more recent estimates, said Dr. Derek Angus, critical care chief at the University of Pittsburgh Medical Center. Although many studies have been reporting death rates based on specific groups such as hospitalized patients, the rate of death among all infected with the coronavirus has been unknown. The news that natural antibodies don’t quickly disappear “will be encouraging for people working on vaccines,” Angus said. Study in NEJM (September 1, 2020): https://doi.org/10.1056/NEJMoa2026116

|
Scooped by
Juan Lama
|
Globally, the COVID-19 pandemic has had extreme consequences for the healthcare system and calls for diagnostic tools to monitor and understand the transmission, pathogenesis and epidemiology, as well as to evaluate future vaccination strategies. Here we have developed novel flexible ELISA-based assays for specific detection of SARS-CoV-2 antibodies against the receptor-binding domain (RBD): An antigen sandwich-ELISA relevant for large population screening and three isotype-specific assays for in-depth diagnostics. Their performance was evaluated in a cohort of 350 convalescent participants with previous COVID-19 infection, ranging from asymptomatic to critical cases. We mapped the antibody responses to different areas on protein N and S and showed that the IgM, A and G antibody responses against RBD are significantly correlated to the disease severity. These assays-and the data generated from them-are highly relevant for diagnostics and prognostics and contribute to the understanding of long-term COVID-19 immunity. Our findings provide support to the notion that antibodies towards SARS-CoV-2 represent a double-edged sword. Antibodies are important in viral neutralization, but also in Fc receptor-mediated phagocytosis, antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cellular cytotoxicity (CDCC) and subsequent elimination of pathogens. However, it is known that particularly ADCC and CDCC can drive harmful and systemic pro-inflammatory responses that can have severe pathophysiological consequences. Thus, based on our findings and others, it may be suggested that an unwanted immune response towards SARS-CoV-2 may be one of the mechanisms causing hyperactivation of macrophages and monocytes, leading to the deadly cytokine storm, which seems to be a hallmark of COVID-19 Preprint of the study available at medRxiv (July 29, 2020): https://doi.org/10.1101/2020.07.27.20162321

|
Scooped by
Juan Lama
|
Antibodies to the virus faded quickly in asymptomatic people, scientists reported. That does not mean immunity disappears. It’s a question that has haunted scientists since the pandemic began: Does everyone infected with the virus produce antibodies — and if so, how long do they last? Not very long, suggests a new study published Thursday in Nature Medicine. Antibodies — protective proteins made in response to an infection — may last only two to three months, especially in people who never showed symptoms while they were infected. The conclusion does not necessarily mean that these people can be infected a second time, several experts cautioned. Even low levels of powerful neutralizing antibodies may still be protective, as are the immune system’s T cells and B cells. But the results offer a strong note of caution against the idea of “immunity certificates” for people who have recovered from the illness, the authors suggested. Antibodies to other coronaviruses, including those that cause SARS and MERS, are thought to last about a year. Scientists had hoped that antibodies to the new virus might last at least as long. Several studies have now shown that most people who are visibly ill with Covid-19 develop antibodies to the virus, although it has been unclear how long those antibodies last. The new study is the first to characterize the immune response in asymptomatic people. The researchers compared 37 asymptomatic people to an equal number who had symptoms in the Wanzhou District of China. The investigators found that asymptomatic people mount a weaker response to the virus than those who develop symptoms. Antibody levels fell to undetectable levels in 40 percent of asymptomatic people, compared with just 13 percent of symptomatic people. The sample size is small, however, and the researchers did not take into account protection offered by immune cells that may fight the virus on their own or make new antibodies when the virus invades. A few studies have shown that the coronavirus stimulates a robust and protective cellular immune response.... Original Study available in Nature Medicine (June 18, 2020): https://doi.org/10.1038/s41591-020-0965-6
|

|
Scooped by
Juan Lama
|
Your body’s antibody response depends on how much sleep you get every night. According to a new study published in the journalCurrent Biology, sleeping for less than six hours is linked to a reduction in antibody response — particularly among men. It could be similar to how Covid-19 vaccine’s effectiveness starts waning around two months after being administered. “The protection conferred by a given vaccine depends on the magnitude of the individual immune response. Antibody response is a clinically significant biomarker of protection and is an early indicator of immunity,” the researchers wrote in their study. So far, studies have identified some factors — like older age, smoking, hypertension, and obesity — that have been associated with a reduced antibody response after getting vaccinated. The researchers decided to look into how sleep patterns could potentially influence a Covid-19 vaccine’s effectiveness because a 2002 study revealed that people with restricted sleep had far lower antibody response following an influenza vaccine compared to those who were sleeping for six to nine hours a day. In subsequent studies, researchers had mixed results while delving into whether fewer hours of sleep could be associated with reduced antibody responses to influenza and hepatitis vaccination. “Our objective is to better inform the scientific community and the public about a relatively easily modifiable behavior that may optimize vaccine response in the context of the current COVID-19 pandemic,” the researchers added in their recent paper. To investigate further, the team analyzed seven studies that looked into people’s sleep duration and hepatitis and influenza vaccines’ effectiveness. However, because there was a lack of data on Covid-19 vaccines specifically, the study highlighted the need to consider simple behavioral interventions like sleep that could improve people’s response to immunization. “While all studies that performed objective sleep assessment were conducted in young and middle-aged subjects, the studies based on self-reported sleep also enrolled 65–85-year-old adults,” the researchers noted. “Given that sleep duration, sleep quality, and vaccination response are generally reduced and more variable in this age range, we performed an exploratory analysis excluding the older age group.” “As suggested by our meta-analysis, adequate amounts of sleep (at least 6 h/night) during the days surrounding the time of vaccination may enhance the humoral response to diverse strains of viruses,” they concluded. The National Sleep Foundation advises adults to sleep for at least seven hours every night up to a maximum of nine hours. And older adults above 65 need seven to eight hours of sleep. “However, large-scale studies are needed (1) to define the time window before and after vaccination where optimizing sleep duration is most likely beneficial,” the researchers added. In a press release, the study’s co-author, Michael Irwin, director of the Cousins Center for Psychoneuroimmunology at the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA said:“We have previously found that cognitive behavioral therapy, as well as mindfulness, robustly improve insomnia and also normalize various aspects of immunity, although it is not yet known whether insomnia treatment can augment vaccination responses.” Anuradha Varanasi is a freelance science writer. She writes on the intersection of health/medicine, racial disparities, and climate change. She earned an MA in Science Journalism from Columbia University in New York City. Research cited published (March 13, 2023) in Current Biology: https://doi.org/10.1016/j.cub.2023.02.017

|
Scooped by
Juan Lama
|
SARS-CoV-2 mRNA booster vaccines provide protection from severe disease, eliciting strong immunity that is further boosted by previous infection. However, it is unclear whether these immune responses are affected by the interval between infection and vaccination. Over a two-month period, we evaluated antibody and B-cell responses to a third dose mRNA vaccine in 66 individuals with different infection histories. Uninfected and post-boost but not previously infected individuals mounted robust ancestral and variant spike-binding and neutralizing antibodies, and memory B cells. Spike-specific B-cell responses from recent infection were elevated at pre-boost but comparatively less so at 60 days post-boost compared to uninfected individuals, and these differences were linked to baseline frequencies of CD27lo B cells. Day 60 to baseline ratio of BCR signaling measured by phosphorylation of Syk was inversely correlated to days between infection and vaccination. Thus, B-cell responses to booster vaccines are impeded by recent infection. Preprint available in medRxiv (August 31, 2022): https://doi.org/10.1101/2022.08.30.22279344

|
Scooped by
Juan Lama
|
Elucidating the dynamics of the neutralizing antibody (nAb) response in coronavirus disease 2019 (COVID-19) convalescents is crucial in controlling the pandemic and informing vaccination strategies. Here we measured nAb titres across 411 sequential plasma samples collected during 1–480 d after illness onset or laboratory confirmation (d.a.o.) from 214 COVID-19 convalescents, covering the clinical spectrum of disease and without additional exposure history after recovery or vaccination against SARS-CoV-2, using authentic SARS-CoV-2 microneutralization (MN) assays. Forty-eight samples were also tested for neutralizing activities against the circulating variants using pseudotyped neutralization assay. Results showed that anti-RBD IgG and MN titres peaked at ~120 d.a.o. and subsequently declined, with significantly reduced nAb responses found in 91.67% of COVID-19 convalescents (≥50% decrease in current MN titres compared with the paired peak MN titres). Despite this decline, majority of the COVID-19 convalescents maintained detectable anti-RBD IgG and MN titres at 400–480 d.a.o., with undetectable neutralizing activity found in 14.41% (16/111) of the mild and 50% (5/10) of the asymptomatic infections at 330–480 d.a.o. Persistent antibody-dependent immunity could provide protection against circulating variants after one year, despite significantly decreased neutralizing activities against Beta, Delta and Mu variants. In conclusion, these data show that despite a marked decline in neutralizing activity over time, nAb responses persist for up to 480 d in most convalescents of symptomatic COVID-19, whereas a high rate of undetectable nAb responses was found in convalescents from asymptomatic infections. A longitudinal analysis of the neutralizing antibody response dynamics in 214 COVID-19 convalescents up to 16 months after infection shows that despite substantial declines in antibody levels over time, they could still provide protection against circulating variants even after one year of infection in most individuals, although neutralizing activities were reduced, particularly against Beta, Delta and Mu SARS-CoV-2 variants. Published in Nat. Microbiology (Feb. 7, 2022): https://doi.org/10.1038/s41564-021-01051-2

|
Scooped by
Juan Lama
|
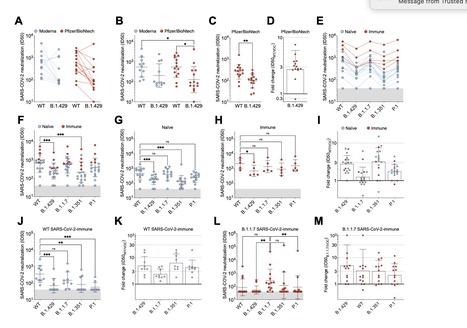
SARS-CoV-2 entry is mediated by the spike (S) glycoprotein which contains the receptor-binding domain (RBD) and the N-terminal domain (NTD) as the two main targets of neutralizing antibodies (Abs). A novel variant of concern (VOC) named CAL.20C (B.1.427/B.1.429) was originally detected in California and is currently spreading throughout the US and 29 additional countries. It is unclear whether antibody responses to SARS-CoV-2 infection or to the prototypic Wuhan-1 isolate-based vaccines will be impacted by the three B.1.427/B.1.429 S mutations: S13I, W152C and L452R. Here, we assessed neutralizing Ab responses following natural infection or mRNA vaccination using pseudoviruses expressing the wildtype or the B.1.427/B.1.429 S protein. Plasma from vaccinated or convalescent individuals exhibited neutralizing titers, which were reduced 3-6 fold against the B.1.427/B.1.429 variant relative to wildtype pseudoviruses. The RBD L452R mutation reduced or abolished neutralizing activity of 14 out of 35 RBD-specific monoclonal antibodies (mAbs), including three clinical-stage mAbs. Furthermore, we observed a complete loss of B.1.427/B.1.429 neutralization for a panel of mAbs targeting the N-terminal domain due to a large structural rearrangement of the NTD antigenic supersite involving an S13I-mediated shift of the signal peptide cleavage site. These data warrant closer monitoring of signal peptide variants and their involvement in immune evasion and show that Abs directed to the NTD impose a selection pressure driving SARS-CoV-2 viral evolution through conventional and unconventional escape mechanisms. Preprint available at bioRxiv (April 1, 2021): https://doi.org/10.1101/2021.03.31.437925

|
Scooped by
Juan Lama
|
New evidence suggests that people who have had COVID-19 may be immune to SARS-CoV-2, the virus that causes it, for at least 5–7 months, if not longer. Recent alleged cases of reinfection with SARS-CoV-2, the coronavirus that causes COVID-19, have raised concerns that the human immune system may only provide short-term protection against the virus. In addition, scarce research has suggested that the number of antibodies in a person’s bloodstream that is capable of disabling the virus declines sharply after an initial infection. However, scientists at the University of Arizona (UArizona) College of Medicine in Tucson have now found evidence of long lasting immunity in people who have had COVID-19. They tested for the presence of antibodies to the virus in nearly 6,000 individuals and then followed them up for several months. “We clearly see high quality antibodies still being produced 5–7 months after SARS-CoV-2 infection,” says Dr. Deepta Bhattacharya, an associate professor of immunobiology at the university, who co-led the research. “Many concerns have been expressed about immunity against COVID-19 not lasting. We used this study to investigate that question and found immunity is stable for at least 5 months.” Bhattacharya points out that people who contracted the SARS-CoV virus responsible for the 2002–2004 outbreak of SARS were still immune 12–17 years after infection. This virus is very similar to SARS-CoV-2. “If SARS-CoV-2 is anything like the first one, we expect antibodies to last at least 2 years, and it would be unlikely for anything much shorter,” he says. In their paper, published in the journal Immunity, the scientists also note that out of nearly 30 million cases of COVID-19 since December 2019, there have been only about 10 confirmed cases of reinfection... Study published in Immunity (Oct. 13, 2020): https://doi.org/10.1016/j.immuni.2020.10.004

|
Scooped by
Juan Lama
|
Long-term antibody responses and neutralizing activities following SARS-CoV-2 infections have not yet been elucidated. We quantified immunoglobulin M (IgM) and G (IgG) antibodies recognizing the SARS-CoV-2 receptor-binding domain (RBD) of the spike (S) or the nucleocapsid (N) protein, and neutralizing antibodies during a period of six months following COVID-19 disease onset in 349 symptomatic COVID-19 patients, which were among the first world-wide being infected. The positivity rate and magnitude of IgM-S and IgG-N responses increased rapidly. High levels of IgM-S/N and IgG-S/N at 2-3 weeks after disease onset were associated with virus control and IgG-S titers correlated closely with the capacity to neutralize SARS-CoV-2. While specific IgM-S/N became undetectable 12 weeks after disease onset in most patients, IgG-S/N titers showed an intermediate contraction phase, but stabilized at relatively high levels over the six months observation period. At late time points the positivity rates for binding and neutralizing SARS-CoV-2-specific antibodies was still over 70%. Taken together, our data indicate sustained humoral immunity in recovered patients who suffer from symptomatic COVID-19, suggesting prolonged immunity. Preprint available at medRxiv (July 24, 2020): https://doi.org/10.1101/2020.07.21.20159178

|
Scooped by
Juan Lama
|
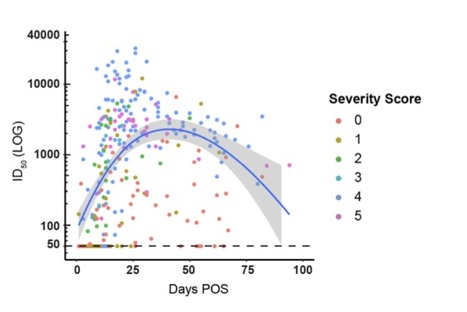
Antibody (Ab) responses to SARS-CoV-2 can be detected in most infected individuals 10-15 days following the onset of COVID-19 symptoms. However, due to the recent emergence of this virus in the human population it is not yet known how long these Ab responses will be maintained or whether they will provide protection from re-infection. Using sequential serum samples collected up to 94 days post onset of symptoms (POS) from 65 RT-qPCR confirmed SARS-CoV-2-infected individuals, we show seroconversion in >95% of cases and neutralizing antibody (nAb) responses when sampled beyond 8 days POS. We demonstrate that the magnitude of the nAb response is dependent upon the disease severity, but this does not affect the kinetics of the nAb response. Declining nAb titres were observed during the follow up period. Whilst some individuals with high peak ID50 (>10,000) maintained titres >1,000 at >60 days POS, some with lower peak ID50 had titres approaching baseline within the follow up period. A similar decline in nAb titres was also observed in a cohort of seropositive healthcare workers from Guy′s and St Thomas′ Hospitals. We suggest that this transient nAb response is a feature shared by both a SARS-CoV-2 infection that causes low disease severity and the circulating seasonal coronaviruses that are associated with common colds. This study has important implications when considering widespread serological testing, Ab protection against re-infection with SARS-CoV-2 and the durability of vaccine protection. Preprint available at medRxiv (July 11, 2020): https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1
|



 Your new post is loading...
Your new post is loading...