Your new post is loading...

|
Scooped by
Juan Lama
|
Neutralizing antibodies that target the BA.4 and BA.5 subvariants were four-fold higher in people aged 55 and older who received the bivalent booster than in those who received a monovalent booster. New data from Pfizer and BioNTech on their bivalent Covid-19 vaccine suggests the updated product may be more protective against more recent Omicron subvariants than the original version of the vaccine, the companies said in a statement released Friday. The companies said the levels of neutralizing antibodies that target the BA.4 and BA.5 subvariants of the SARS-CoV-2 virus were four-fold higher in people aged 55 and older who received the bivalent booster than in similarly aged people who received a monovalent booster. The bivalent, which was given an emergency use authorization at the end of August, targets both the original version of the SARS-2 virus and the BA.4/BA.5 variants. Recently BA.5 has been the dominant strain in the United States, but an alphabet soup of newer subvariants — BA.4.6, BQ.1.1 among them — is starting to crowd it out. The new data from the companies only looks at what getting the the booster did to antibody levels in recipients. The trial did not test whether people who received the updated boosters were less likely to contract Covid than people who received one of the older boosters. “These data demonstrate that our BA.4/BA.5-adapted bivalent vaccine works as conceptually planned in providing stronger protection against the Omicron BA.4 and BA.5 sublineages,” Ugur Sahin, CEO and co-founder of BioNTech, said in the statement. “In the next step and as part of our science-based approach, we will continue to evaluate the cross-neutralization of the adapted vaccine against new variants and sublineages. Our aim is to provide broader immunity against Covid-19 caused by SARS-CoV-2, including Omicron and other circulating strains.” The companies also reported that one month after the trial participants got a dose of the bivalent booster, neutralizing antibodies targeting Omicron BA.4/BA.5 viruses increased 13.2-fold from pre-booster levels in adults who were older than 55 years of age; they increased 9.5-fold for adults 18 to 55 years of age. By comparison, in adults older than 55 who received a booster dose of the original vaccine, antibody titers to BA.4 and BA.5 rose 2.9-fold over the same period. Of late there have been a number of small studies that have tried to get an answer to the question of whether the updated vaccines are likely to be more protective than the original version against Omicron viruses. Three concluded that updating the vaccine did not make a difference while two suggested there was a benefit. But differences in the designs of the studies make them hard to compare to each other and to the Pfizer data. And at the end of the day, the important question is whether what was seen in terms of antibody production will translate into better protection for people who receive the bivalent vaccine, said Florian Krammer, a vaccinologist at Mount Sinai School of Medicine in Manhattan. He thought it might.
“A four-fold higher titer, that would be good,” said Krammer, who has done some paid consulting work for Pfizer. “Four-fold is usually the magical cut-off for a lot of us when we look at neutralization. Four-fold seems to mean something.” He cautioned though, that with different groups coming up with different estimates of whether the bivalent vaccine generated a significant improvement in antibody levels, “we really need to figure out what the truth is here and who is right in terms of measuring.” Eric Topol, director of the Scripps Research Translational Institute, thought the results were promising. “I think this is encouraging,” Topol told STAT. “We just need more people to get the darn booster.” Data from the Centers for Disease Control show uptake of the bivalent boosters has been slow, with only 26.3 million people having received it so far. Even in the highest risk age group, people 65 and older, uptake has been modest. To date only 23% of Americans in that age group have received a bivalent booster. Pfizer press release (Nov. 04, 2022) available here: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-updated-clinical-data-omicron

|
Scooped by
Juan Lama
|
The B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has splintered into multiple subvariants with increased transmissibility and immune escape.1 At the time of this report, omicron subvariant BA.5 is the dominant global virus and has shown substantial immune escape as compared with previous omicron subvariants.2-5 BA.4.6 is a sublineage of BA.4 with two additional mutations in the spike protein (R346T and N658S) (Figure 1A) and has recently increased in prevalence in certain regions currently dominated by BA.5, including in the United States. The ability of BA.4.6 to evade neutralizing antibodies that were induced by infection or vaccination remains to be determined. We evaluated neutralizing antibody titers against five SARS-CoV-2 strains — WA1/2020 and omicron subvariants BA.1, BA.2, BA.4–BA.5, and BA.4.6 — in 19 participants who had been recently infected with the omicron BA.1 or BA.2 subvariant and in 16 participants who had been vaccinated and boosted with the original mRNA-1273 vaccine (Moderna) (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). In the cohort with previous omicron infection, all the participants except for one had been vaccinated; samples were obtained a median of 21 days after diagnosis of omicron infection. In this cohort, the median pseudovirus neutralizing antibody titer was 42,067 against WA1/2020, 6352 against BA.1, 3854 against BA.2, 1673 against BA.4–BA.5, and 630 against BA.4.6 (Figure 1B). The median neutralizing antibody titers against BA.4.6 were lower than the median titers against WA1/2020 by a factor of 67, against BA.1 by a factor of 10, against BA.2 by a factor of 6, and against BA.4–BA.5 by a factor of 2.7. In the mRNA-1273 vaccine cohort, participants were excluded if they had a known history of SARS-CoV-2 infection or positive results on nucleocapsid serologic analysis or if they had received immunosuppressive medications or other vaccines against SARS-CoV-2. Six months after the initial two mRNA-1273 immunizations, the median neutralizing antibody titer was 951 against WA1/2020, 28 against BA.2, 30 against BA.4–BA.5, and 23 against BA.4.6 (Figure 1C). At a median of 17 days after the first booster dose, the median neutralizing antibody titer was 16,011 against WA1/2020, 802 against BA.2, 449 against BA.4–BA.5, and 225 against BA.4.6. The median neutralizing antibody titer against BA.4.6 was lower than that against WA1/2020 by a factor of 71, against BA.2 by a factor of 4, and against BA.4–BA.5 by a factor of 2. Our data show that the BA.4.6 omicron subvariant markedly escaped neutralizing antibodies induced by infection or vaccination, with values that were lower than BA.5 titers by a factor of 2 to 2.7, which suggests continued evolution of SARS-CoV-2. These findings provide immunologic context for the increasing prevalence of BA.4.6 in populations in which BA.5 is currently dominant. Moreover, the R346T mutation had also recently been observed in other omicron subvariants, including BA.2.75 and BA.5, which suggests the biologic relevance of this mutation. The potential effect of the emergence of the BA.4.6 subvariant on vaccine boosters containing BA.5 immunogens or on infection with BA.5 remains to be determined. Published in NEJM (Oct. 19, 2022): https://doi.org/10.1056/NEJMc2212117

|
Scooped by
Juan Lama
|
A top F.D.A. regulator cited compelling data for redesigned coronavirus vaccines from Pfizer-BioNTech and Moderna. WASHINGTON — The Biden administration plans to offer the next generation of coronavirus booster shots to Americans 12 and older soon after Labor Day, a campaign that federal officials hope will reduce deaths from Covid-19 and protect against an expected winter surge. Dr. Peter Marks, the top vaccine regulator for the Food and Drug Administration, said in an interview on Tuesday that while he could not discuss timing, his team was close to authorizing updated doses that would target the versions of the virus now circulating. Even though those formulations have not been tested in humans, he said, the agency has “extremely good” data showing that the shots are safe and will be effective. “How confident am I?” he said. “I’m extremely confident.” This week, both Moderna and Pfizer-BioNTech finalized their submissions to the F.D.A. asking for emergency authorization of booster shots aimed at BA.5 and another subvariant of Omicron that together account for most coronavirus cases in the United States. Federal health officials say they are eager to offer the updated boosters as quickly as possible, pointing to a death toll that now averages about 450 Americans per day and could rise in the coming months as people spend more time indoors. “We have really got to do better to protect the American public,” Dr. Anthony S. Fauci, President Biden’s chief medical adviser, said in an interview on Tuesday. “We are in the middle of a BA.5 outbreak here, and we are nowhere near where we want to be.” The Biden administration has struggled to convince Americans of the need for successive vaccinations. Only about two-thirds of the population has been inoculated with the primary series of two shots, and far fewer have received booster doses. Some outside scientists have said the government is moving too fast to clear redesigned shots, arguing that the existing vaccines provide strong protection against severe disease. “Deaths are concentrated in unvaccinated people and people with serious health conditions,” said John P. Moore, a virologist at Weill Cornell Medicine. He said the extra protection that the new shots would provide against infection could be “weak to nonexistent.” Jeremy Kamil, a virologist at Louisiana State University Health Shreveport, said that although he supported new boosters, many people had immunity because of recent infections. “Even if we get this out in the next 10 days, how many people are left who haven’t gotten Omicron?” he said. Other scientists said that the government’s plan made sense given how the virus had changed and the evidence that immunity wanes over time. Dr. Marks said that if regulators waited for additional data or recommendations from outside experts, the virus might evolve further and “we may have lost a bunch of individuals who could otherwise be sitting around at the dinner table together.” In a sign of impending action from the F.D.A., the Centers for Disease Control and Prevention has scheduled a two-day meeting of its advisory panel of experts on the matter for Sept. 1 and 2. The C.D.C. director, Rochelle P. Walensky, would then make a final decision on whether to roll out the new doses. Shipments to states could begin as early as next week, according to officials familiar with the plan. The government plans to offer the new Pfizer booster to everyone 12 and older while limiting the new Moderna shot to adults. People who have already received the initial two-shot series of either vaccine would be eligible. So would those who have received the initial shots plus one or two booster shots. The new booster campaign could be broadened to younger children later. Dr. Marks suggested that the biggest obstacle to the effort was the level of complacency that had set in, even as the pandemic continued to exact what he called an “unacceptable” death toll. He said the F.D.A. might recommend that people who had recently received a Covid vaccine dose wait “a few months” before getting the new shot, even if they were otherwise eligible. He said the C.D.C. might weigh in on whether people who were recently infected with the virus should also wait. As of mid-August, the federal government had bought more than 170 million doses of the updated version of the vaccines. This month, the C.D.C. laid out detailed plans to offer the shots, warning that the supply would be “sufficient but finite” and saying that doses should be “directed to providers with expected demand among eligible patients.” The new shots combine the original vaccine with components aimed at the BA.4 and BA.5, Omicron’s recent subvariants. Officials argue that the new formulations will deliver a stronger boost to the immune system than the existing vaccines provide. Unlike earlier shots, the redesigned formulations have not been tested widely on humans; instead, the companies have submitted data from mice trials. Some vaccine experts have complained that animal data is too preliminary and say regulators should wait for results of human clinical trials. But Dr. Fauci said using animal data was “not anything different than we always do” in updating the flu vaccine each year. Dr. Marks said other evidence included the extensive track record with the existing vaccines and a series of earlier human trials with variant-specific formulations. “I take great issue with those who say, ‘Oh, you’re just approving this with mouse data,’” he said. “We’re authorizing this with the totality of the evidence that we have.” Moderna and Pfizer have both submitted clinical data from human studies of redesigned shots targeting the original version of the Omicron variant. Britain last week authorized that version of Moderna’s vaccine, but U.S. regulators asked for formulations aimed at Omicron’s newer subvariants. Researchers are still working to answer key questions about the protection that the new vaccines deliver, including the levels of antibodies the shots generate in humans and how those antibodies protect people. Moderna began human trials of its new vaccine this month, and Pfizer plans to do the same later in the month. Initial data from those trials is expected later this year. Dr. Moore, the virologist at Weill Cornell Medicine, said the administration’s plans could backfire if the fall or winter brings a wave of disease despite the new boosters, potentially reducing overall confidence in Covid-19 vaccines. “My issue all along has been: Is there enough data to really justify the effort?” Dr. Moore said. “The potential downside is, if the public thinks that this Omicron-containing booster is some kind of magic bullet that will give them superstrong protection from infection, is there a risk that they will change their behavior to increase their exposure?” The F.D.A. will decide whether to authorize the retooled doses without seeking a recommendation from its outside advisory panel of experts, a step it usually takes before making new vaccines available. Critics have complained that regulators have bypassed the panel at crucial steps. Dr. Marks defended the decision, saying a late June meeting of the advisory panel on the need to revise the vaccines had given regulators “everything we needed.” The committee voted overwhelming then in favor of updating the vaccines to work better against Omicron or its subvariants, but it did not consider specific formulations.

|
Scooped by
Juan Lama
|
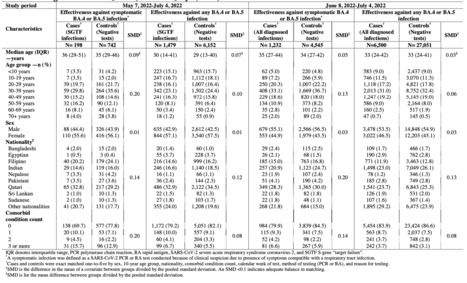
This study estimates the effectiveness of previous infection with SARSCoV2 in preventing reinfection with Omicron BA.4/BA.5 subvariants using a test negative, case control study design. Cases (SARSCoV2 positive test results) and controls (SARSCoV2 negative test results) were matched according to sex, 10 year age group, nationality, comorbid condition count, calendar week of testing, method of testing, and reason for testing. Effectiveness was estimated using the S gene target failure (SGTF) infections between May 7, 2022 and July 4, 2022. SGTF status provides a proxy for BA.4/BA.5 infections, considering the negligible incidence of other SGTF variants during the study. Effectiveness was also estimated using all diagnosed infections between June 8, 2022 and July 4, 2022, when BA.4/BA.5 dominated incidence. Effectiveness of a previous pre-Omicron infection against symptomatic BA.4/BA.5 reinfection was 15.1% (95% CI: -47.1 to 50.9%), and against any BA.4/BA.5 reinfection irrespective of symptoms was 28.3% (95% CI: 11.4 to 41.9%). Effectiveness of a previous Omicron infection against symptomatic BA.4/BA.5 reinfection was 76.1% (95% CI: 54.9 to 87.3%), and against any BA.4/BA.5 reinfection was 79.7% (95% CI: 74.3 to 83.9%). Results using all diagnosed infections when BA.4/BA.5 dominated incidence confirmed the same findings. Sensitivity analyses adjusting for vaccination status confirmed study results. Protection of a previous infection against BA.4/BA.5 reinfection was modest when the previous infection involved a preOmicron variant, but strong when the previous infection involved the Omicron BA.1 or BA.2 subvariants. Protection of a previous infection against BA.4/BA.5 was lower than that against BA.1/BA.2, consistent with BA.4/BA.5 greater capacity for immune system evasion than that of BA.1/BA.2. Preprint available at medRxiv (July 12, 2022): https://doi.org/10.1101/2022.07.11.22277448

|
Scooped by
Juan Lama
|
Probably many people who watched or participated in the June 28 virtual US Food and Drug Administration (FDA) advisory committee meeting about updating COVID-19 vaccines could agree on 1 point, made by the agency’s Peter Marks, MD, PhD: “It is science at its hardest.” The FDA convened its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss whether to add an Omicron component to boosters for the fall. In order to have enough doses by early October, “we will need to very rapidly move to let companies know what that selection will be,” Marks reminded the panelists. (How many doses will be enough isn’t clear—as of June 30, only 51.1% of fully vaccinated US adults aged 18 years or older had received 1 booster shot, while only 27% of fully vaccinated adults aged 50 years or older, for whom a second booster is recommended, had received 2, according to government data.) Omicron, which the World Health Organization (WHO) classified as a variant of concern (VOC) in November 2021, is the first VOC that can evade the immune system, resulting in lower vaccine effectiveness, the WHO’s Kanta Subbarao, MBBS, MPH, told committee members. Even so, she noted, after a booster dose, the available prototype vaccines, which are based on the ancestral SARS-CoV-2 index virus strain that has long been undetectable among circulating viruses, continue to protect people against serious illness and death. After a day of listening to presentations by Subbarao, director of the WHO’s Collaborating Centre for Reference and Research on Influenza in Melbourne, and scientists from the FDA, the Centers for Disease Control and Prevention (CDC), and 3 vaccine manufacturers—Moderna, Pfizer, and Novavax—the advisory committee voted 19-2 to recommend inclusion of a SARS-CoV-2 Omicron component for COVID-19 booster vaccines this fall. Less than 48 hours later, the FDA, which usually follows advisory committee recommendations after such lopsided votes, announced that manufacturers seeking to update COVID-19 vaccines should add a spike protein component of the Omicron subvariants BA.4 and BA.5 (which differ only outside the spike protein) to their prototype vaccines to make bivalent boosters that can be used beginning this fall. “[W]e have not advised manufacturers to change the vaccine for primary vaccination, since a primary series with the FDA-authorized and approved COVID-19 vaccines provide a base of protection against serious outcomes of COVID-19 caused by circulating strains of SARS-CoV-2,” Marks, director of the agency’s Center for Biologics Evaluation and Research, said in the announcement. Yet the data presented at the VRBPAC meeting were about experimental bivalent boosters that combined prototype vaccines with the spike protein of the original Omicron variant, BA.1. When the advisory committee reconvened after lunch, Marks noted that in the week ending June 25, BA.4 and BA.5 represented more than half of US circulating SARS-CoV-2 variants for the first time, according to newly released CDC data. By the week ending July 2, BA.5 alone represented 53.6% of US circulating SARS-CoV-2 variants, while BA.4 represented 16.5%, according to the CDC. CDC data show that BA.1, which represented about a third of circulating US variants on March 26, was undetectable by May 21—an illustration of just how quickly SARS-CoV-2 is evolving.....

|
Scooped by
Juan Lama
|
BA.5 has become dominant in the U.S. In June, the Centers for Disease Control and Prevention reported that the Omicron subvariants BA.4 and BA.5 had become dominant in the U.S. The agency revised that statement on July 5 to say that BA.5 made up over half of new cases in the country. Experts said that, in general, these subvariants do not have markedly divergent symptoms from earlier versions of Omicron. People infected with BA.4 and BA.5 may develop a cough, runny nose, sore throat, fatigue, headaches and muscle pains. However, they are less likely to lose their senses of taste and smell, or to experience shortness of breath, as compared with those infected with Delta or other variants of the coronavirus, said Dr. Peter Chin-Hong, an infectious disease specialist at the University of California, San Francisco. Dr. Joseph Khabbaza, a pulmonary and critical care physician at Cleveland Clinic, said people tend to experience upper respiratory symptoms “from the vocal cords to the tip of the nose.” Anecdotally, he said, he has seen more patients with painful sinus congestion and severe sore throats who have tested positive for Covid-19 while BA.4 and BA.5 have been circulating. Some of them thought they had strep throat because they were in so much pain, he said. There also is not yet evidence to indicate that these subvariants cause more severe disease than past versions of Omicron. But BA.4 and BA.5 are more contagious, which means that as more people become infected, both adult and pediatric hospitalizations are increasing, said Dr. Adam Ratner, director of the division of pediatric infectious diseases at Hassenfeld Children’s Hospital at N.Y.U. Langone.

|
Scooped by
Juan Lama
|
Vaccines and prior infection still prevent severe disease from new SARS-CoV-2 strains. Once again, South Africa is at the forefront of the changing COVID-19 pandemic. Epidemiologists and virologists are watching closely as cases there rise sharply again, just 5 months after the Omicron variant caused a dramatic surge. This time, the drivers are two new subvariants of Omicron named BA.4 and BA.5, which the Network for Genomic Surveillance in South Africa first detected in January. The new strains didn’t have much of an impact initially, but over the past few weeks case numbers in South Africa jumped from roughly 1000 per day on 17 April to nearly 10,000 on 7 May. A third subvariant called BA.2.12.1 is spreading in the United States, driving increases along the East Coast. It’s still unclear whether the new subvariants will cause another global COVID-19 wave. But like the earlier versions of Omicron, they have a remarkable ability to evade immunity from vaccines, previous infection, or both—a disturbing portent for the future of the pandemic and a potentially serious complication for vaccine developers. In most cases, vaccination or earlier infection still seem to provide protection from severe disease. “There’s no reason to freak out,” says John Moore, an immunologist at Weill Cornell Medicine. The new strains are “an additional hassle,” he says, but “there’s no indication that they’re more dangerous or more pathogenic.” Hospitalizations in South Africa, for example, have increased, “but because it is starting from a very low level, it’s not cause for alarm,” says virologist Tulio de Oliveira of Stellenbosch University, who helped identify BA.4 and BA.5. Numbers of patients in intensive care units are as low as they have been since the start of the pandemic, he says. “At the moment, we expect something similar to the Omicron BA.1 wave,” when hospitalization rates stayed manageable. The new superspreaders do, however, showcase the restless virus’ ability to find ways around the “immunity wall” built up over the past 2 years and to continue to circulate at high levels. Even if the new variants cause relatively little severe disease, “it’s a numbers game,” says Leif Erik Sander, an infectious disease expert at the Charité University Hospital in Berlin; enough new infections could still overwhelm health systems. All three new strains share key mutations with the BA.2 strain of Omicron, which, like BA.1, emerged in southern Africa in October 2021. Initial studies by de Oliveira and Alex Sigal, an infectious disease expert at the Africa Health Research Institute in Durban, suggest BA.4 and BA.5 can elude the immunity of patients who were infected with the BA.1 strain, which in South Africa caused a much larger wave than BA.2. That may be in part because immunity has waned since South Africa’s BA.1 wave peaked in December. People who were both vaccinated and infected had somewhat stronger protection, de Oliveira and Sigal reported in a 2 May preprint. All three new variants have mutations that alter a key amino acid called L452, which may help explain their ability to dodge immunity. L452 is part of the receptor-binding domain, the part of the spike protein that locks onto cells, enabling infection. The domain is also a key target for protective antibodies. The Delta variant that caused devastating surges around the world in 2021 had mutations in L452 as well, so many scientists have been watching this hot spot carefully, including immunologist Yunlong Richard Cao of Peking University. On 11 April, Cao says, he and his colleagues noticed a pattern: New Omicron sublineages from New York, Belgium, France, and South Africa all had changes in L452. “The independent appearance of four different mutations at the same site? That’s not normal,” Cao says. The researchers suspected it was the virus’ response to the high levels of immunity generated by the huge Omicron waves. They immediately started to make copies of the spike protein based on the new sequences and test how well different antibodies could block those proteins, preventing them from binding to cells. They used sera from 156 vaccinated and boosted subjects, including some who had recovered from either BA.1 or severe acute respiratory syndrome (SARS), the coronavirus disease that caused a deadly global outbreak almost 2 decades ago. Like the South African team, they found that blood from patients who had been infected with BA.1 had only weak ability to neutralize BA.4 and BA.5; the same was true for BA.2.12.1. Even less effective were sera from people who had previously been infected with SARS and then vaccinated against COVID-19, they reported in a 2 May preprint. The latter finding was surprising. Previous work by Linfa Wang, a bat coronavirus researcher at the Duke-NUS Medical School in Singapore, had shown patients who had recovered from SARS and were then vaccinated had strong protection against earlier SARS-CoV-2 variants—and even some related animal viruses—a finding that seemed to hold clues to developing vaccines effective against multiple coronaviruses, including those that might trigger the next pandemic. But the new mutations apparently helped the Omicron subvariants evade those previously powerful antibodies. Wang notes, however, that the subjects in the new study were all vaccinated with CoronaVac, a Chinese vaccine made from inactivated virus. Subjects in his study were vaccinated with messenger RNA (mRNA) vaccines, which might provide a more potent response to the new strains, he says. But Wang agrees that Omicron’s knack for immune escape is dramatic. Based on its immunological profile, it “should be called SARS-3,” he says—an entirely distinct virus. Omicron’s rapid evolution creates difficult decisions for vaccine- and policymakers about whether to shift to a new set of vaccines or stick with the current formulations, which are based on the virus that emerged in Wuhan, China, more than
2 years ago. Moderna has tested two versions of its mRNA vaccine, containing the ancestral strain and either the Beta variant—which spread in South Africa for a while in 2021 but is now gone—or the Omicron BA.1 variant. The company has not yet reported data on how well they might protect against the new subvariants. Pfizer, the other mRNA vaccine producer, has tested the efficacy of a booster and a primary vaccine based on BA.1. Results are expected by the end of June. The U.S. Food and Drug Administration has scheduled a meeting for 28 June to analyze available data and make vaccine recommendations for the fall. The limited protection that BA.1 infection provided against the new subvariants in lab studies has already raised questions about how useful the new Omicron-specific vaccines might be. Wang says the virus is evolving too quickly for strain-specific vaccines to keep up. Instead, a broad cocktail of monoclonal antibodies targeting different strains might be the best way forward, he says. Such a shot could prevent infections for several months in those vulnerable to severe disease, including immunocompromised people who don’t respond to vaccines. Protecting that group is crucial, he notes, because many researchers suspect new variants emerge during long-term infections in people whose immune systems fail to clear the virus. The main hurdle, Wang says, is cost: A dose of monoclonal antibodies is about $1000 per patient, he notes, “but if someone could find a way to lower that to $50 or $100,” the approach could be cheaper than constantly updating vaccines. Kristian Andersen, who studies viral evolution at Scripps Research, draws a sobering lesson from the newest Omicron variants. Although we don’t know what future variants will look like, he says, “we can be certain that they’ll continue to be more and more capable of immune escape,” possibly leading to lower protection against not just infection, but also against severe disease. “We need to focus on broadening our immunity,” he says. It’s far from clear what kind of vaccine might prompt that broadened immunity, but “we really, really need to get going” to figure that out, Andersen says. “Simply letting the virus do what viruses do—continue to infect us, and likely several times a year—just isn’t an option in my playbook.” Published in Science (May 10, 2022): https://doi.org/10.1126/science.adc9473

|
Scooped by
Juan Lama
|
Recent emergence of SARS-CoV-2 Omicron sublineages BA.2.12.1, BA.2.13, BA.4 and BA.5 all contain L452 mutations and show potential higher transmissibility over BA.2. The new variants' receptor binding and immune evasion capability require immediate investigation, especially on the role of L452 substitutions. Herein, coupled with structural comparisons, we showed that BA.2 sublineages, including BA.2.12.1 and BA.2.13, exhibit increased ACE2-binding affinities compared to BA.1; while BA.4/BA.5 shows the weakest receptor-binding activity due to F486V and R493Q reversion. Importantly, compared to BA.2, BA.2.12.1 and BA.4/BA.5 exhibit stronger neutralization escape from the plasma of 3-dose vaccinees and, most strikingly, from vaccinated BA.1 convalescents. To delineate the underlying evasion mechanism, we determined the escaping mutation profiles, epitope distribution and Omicron sublineage neutralization efficacy of 1640 RBD-directed neutralizing antibodies (NAbs), including 614 isolated from BA.1 convalescents. Interestingly, post-vaccination BA.1 infection mainly recalls wildtype (WT) induced humoral memory and elicits antibodies that neutralize both WT and BA.1. These cross-reactive NAbs are significantly enriched on non-ACE2-competing epitopes; and surprisingly, the majority are undermined by R346 and L452 substitutions, namely R346K (BA.1.1), L452M (BA.2.13), L452Q (BA.2.12.1) and L452R (BA.4/BA.5), suggesting that R346K and L452 mutations appeared under the immune pressure of Omicron convalescents. Nevertheless, BA.1 infection can also induce new clones of BA.1-specific antibodies that potently neutralize BA.1 but do not respond to WT SARS-CoV-2, due to the high susceptibility to N501, N440, K417 and E484. However, these NAbs are largely escaped by BA.2 sublineages and BA.4/BA.5 due to D405N and F486V, exhibiting poor neutralization breadths. As for therapeutic NAbs, LY-CoV1404 (Bebtelovimab) and COV2-2130 (Cilgavimab) can still effectively neutralize BA.2.12.1 and BA.4/BA.5, while the S371F, D405N and R408S mutations carried by BA.2/BA.4/BA.5 sublineages would undermine most broad sarbecovirus NAbs. Together, our results indicate that Omicron can evolve mutations to specifically evade humoral immunity elicited by BA.1 infection. The continuous evolution of Omicron poses great challenges to SARS-CoV-2 herd immunity and suggests that BA.1-derived vaccine boosters may not be ideal for achieving broad-spectrum protection. Preprint available in bioRxiv (May 02, 2022): https://doi.org/10.1101/2022.04.30.489997
|

|
Scooped by
Juan Lama
|
The SARS-CoV-2 Omicron variant and its numerous sub-lineages have exhibited a striking ability to evade humoral immune responses induced by prior vaccination or infection. The Food and Drug Administration (FDA) has recently granted Emergency Use Authorizations (EUAs) to new bivalent formulations of the original Moderna and Pfizer mRNA SARS-CoV-2 vaccines that target both the ancestral strain as well as the Omicron BA.4/BA.5 variant. Despite their widespread use as a vaccine boost, little is known about the antibody responses induced in humans. Here, we collected sera from several clinical cohorts: individuals after three or four doses of the original monovalent mRNA vaccines, individuals receiving the new bivalent vaccines as a fourth dose, and individuals with BA.4/BA.5 breakthrough infection following mRNA vaccination. Using pseudovirus neutralization assays, these sera were tested for neutralization against an ancestral SARS-CoV-2 strain, several Omicron sub-lineages, and several related sarbecoviruses. At ~3-5 weeks post booster shot, individuals who received a fourth vaccine dose with a bivalent mRNA vaccine targeting BA.4/BA.5 had similar neutralizing antibody titers as those receiving a fourth monovalent mRNA vaccine against all SARS-CoV-2 variants tested, including BA.4/BA.5. Those who received a fourth monovalent vaccine dose had a slightly higher neutralizing antibody titers than those who received the bivalent vaccine against three related sarbecoviruses: SARS-CoV, GD-Pangolin, and WIV1. When given as a fourth dose, a bivalent mRNA vaccine targeting Omicron BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not induce superior neutralizing antibody responses in humans, at the time period tested, compared to the original monovalent vaccine formulation. Preprint available in bioRxiv (Oct. 24, 2022): https://www.biorxiv.org/content/10.1101/2022.10.22.513349v1 https://doi.org/10.1101/2022.10.22.513349

|
Scooped by
Juan Lama
|
Planas et al analyze the extent and duration of the neutralizing antibody response following vaccination with Pfizer BNT162b2 mRNA in the sera and nasal swabs from individuals with or without Omicron breakthrough infection, finding a short duration of neutralization against BA.5 after boosting and strong IgA response upon breakthrough infection. Background Since early 2022, Omicron BA.1 has been eclipsed by BA.2, which was in turn outcompeted by BA.5, that displays enhanced antibody escape properties. Methods Here, we evaluated the duration of the neutralizing antibody (Nab) response, up to 18 months after Pfizer BNT162b2 vaccination, in individuals with or without BA.1/BA.2 breakthrough infection. We measured neutralization of the ancestral D614G lineage, Delta and Omicron BA.1, BA.2, BA.5 variants in 300 sera and 35 nasal swabs from 27 individuals. Findings Upon vaccination, serum Nab titers were reduced by 10-, 15- and 25-fold for BA.1, BA.2 and BA.5, respectively, compared with D614G. We estimated that after boosting, the duration of neutralization was markedly shortened from 11.5 months with D614G to 5.5 months with BA.5. After breakthrough, we observed a sharp increase of Nabs against Omicron subvariants, followed by a plateau and a slow decline after 5-6 months. In nasal swabs, infection, but not vaccination, triggered a strong IgA response and a detectable Omicron neutralizing activity. Conclusions Thus, BA.5 spread is partly due to abbreviated vaccine efficacy, particularly in individuals who were not infected with previous Omicron variants. Published in Med (October 5, 2022)

|
Scooped by
Juan Lama
|
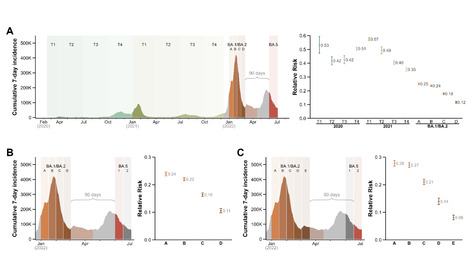
SARS-CoV-2 omicron subvariants BA.1 and BA.2 became dominant in many countries in early 2022. These subvariants are now being displaced by BA.4 and BA.5. While natural infection with BA.1/BA.2 provides some protection against BA.4/BA.5 infection, the duration of this protection remains unknown. We used the national Portuguese COVID-19 registry to investigate the waning of protective immunity conferred by prior BA.1/BA.2 infection towards BA.5. We divided the individuals infected during the period of BA.1/BA.2 dominance (>90% of sample isolates) in successive 15-day intervals and determined the risk of subsequent infection with BA.5 over a fixed period. Compared with uninfected people, one previous infection conferred substantial protection against BA.5 re-infection at 3 months (RR=0.12; 95% CI: 0.11-0.12). However, although still significant, the protection was reduced by two-fold at 5 months post-infection (RR=0.24; 0.23-0.24). These results should be interpreted in the context of vaccine breakthrough infections, as the vaccination coverage in the individuals included in the analyses is >98% since the end of 2021. This waning of protection following BA.1/BA.2 infection highlights the need to assess the stability and durability of immune protection induced with the adapted vaccines (based on BA.1) over time. Preprint available at medRxiv (August 17, 2022): https://doi.org/10.1101/2022.08.16.22278820

|
Scooped by
Juan Lama
|
Genetically distinct viral variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been recorded since January 2020. Over this time global vaccine programs have been introduced, contributing to lowered COVID-19 hospitalisation and mortality rates, particularly in developed countries. In late 2021, the Omicron BA.1 variant emerged, with significant genetic differences and clinical effects from other variants of concern (VOC). This variant demonstrated higher numbers of polymorphisms in the gene encoding the Spike (S) protein, and there has been displacement of the dominant Delta variant. Shortly after dominating global spread in early 2022, BA.1 was supplanted by the genetically distinct Omicron lineage BA.2. A sub-lineage of BA.2, designated BA.5 has now started to dominate globally, with the potential to supplant BA.2. To address the relative threat of BA.5, we determined infectivity to particle ratios in primary nasopharyngeal samples and expanded low passage isolates in a well characterised, genetically engineered ACE2/TMPRSS2 cell line. We then assessed the impact of BA.5 infection on humoral neutralisation in vitro, in vaccinated and convalescent cohorts, using concentrated human IgG pooled from thousands of plasma donors, and licensed monoclonal antibody therapies. The infectivity of virus in primary swabs and expanded isolates revealed whilst BA.1 and BA.2 are attenuated through ACE2/TMPRSS2, BA.5 infectivity is equivalent to that of an early 2020 circulating clade and has greater sensitivity to the TMPRSS2 inhibitor Nafamostat. As with BA.1, we observed BA.5 to significantly reduce neutralisation titres across all donors. Concentrated pooled human IgG from convalescent and vaccinated donors had greater breadth of neutralisation, although the potency was still reduced 7-fold with BA.5. Of all therapeutic antibodies tested, we observed a 14.3-fold reduction using Evusheld and 16.8 reduction using Sotrovimab when neutralising a Clade A versus BA.5 isolate. These results have implications for ongoing tracking and management of Omicron waves globally. Preprint available in medRxiv (JUly 10, 2022): https://doi.org/10.1101/2022.07.07.22277128

|
Scooped by
Juan Lama
|
The latest omicron offshoot, BA.5, has quickly become dominant in the United States, driving a wave of reinfections across the country. America has decided the pandemic is over. The coronavirus has other ideas. The latest omicron offshoot, BA.5, has quickly become dominant in the United States, and thanks to its elusiveness when encountering the human immune system, is driving a wave of cases across the country. The size of that wave is unclear because most people are testing at home or not testing at all. The Centers for Disease Control and Prevention in the past week has reported a little more than 100,000 new cases a day on average. But infectious-disease experts know that wildly underestimates the true number, which may be as many as a million, said Eric Topol, a professor at Scripps Research who closely tracks pandemic trends. Antibodies from vaccines and previous covid infections offer limited protection against BA.5, leading Topol to call it “the worst version of the virus that we’ve seen.” Other experts point out that, despite being hit by multiple rounds of ever-more-contagious omicron subvariants, the country has not yet seen a dramatic spike in hospitalizations. About 38,000 people were hospitalized nationally with covid as of Friday, according to data compiled by The Washington Post. That figure has been steadily rising since early March, but remains far below the record 162,000 patients hospitalized with covid in mid-January. The average daily death toll on Friday stood at 329 and has not changed significantly over the past two months. There is widespread agreement among infectious-disease experts that this remains a dangerous virus that causes illnesses of unpredictable severity — and they say the country is not doing enough to limit transmission. Restrictions and mandates are long gone. Air travel is nearly back to pre-pandemic levels. Political leaders aren’t talking about the virus — it’s virtually a nonissue on the campaign trail. Most people are done with masking, social distancing, and the pandemic generally. They’re taking their chances with the virus. “It’s the wild west out there,” said Ziyad Al-Aly, an epidemiologist at Washington University in St. Louis. “There are no public health measures at all. We’re in a very peculiar spot, where the risk is vivid and it’s out there, but we’ve let our guard down and we’ve chosen, deliberately, to expose ourselves and make ourselves more vulnerable.” Angela Rasmussen, a virologist at the University of Saskatchewan, would like to see more money for testing and vaccine development, as well as stronger messaging from the Biden administration and top health officials. She was dismayed recently on a trip to southern California, where she saw few people wearing masks in the airport. “This is what happens when you don’t have politicians and leaders taking a strong stand on this,” she said. The CDC said it has urged people to monitor community transmission, “stay up to date on vaccines, and take appropriate precautions to protect themselves and others....”

|
Scooped by
Juan Lama
|
The lineages’ rise seems to stem from their ability to infect people who were immune to earlier forms of Omicron and other variants. Like a Hollywood franchise that churns out sequel after mind-numbing sequel, Omicron is back. Mere weeks after the variant’s BA.2 lineage caused surges globally, two more Omicron spin-offs are on the rise worldwide. First spotted by scientists in South Africa in April and linked to a subsequent rise in cases there, BA.4 and BA.5 are the newest members of Omicron’s growing family of coronavirus subvariants. They have been detected in dozens of countries worldwide. The BA.4 and BA.5 subvariants are spiking globally because they can spread faster than other circulating variants — mostly BA.2, which caused a surge in cases at the beginning of the year. But so far, the latest Omicron variants seem to be causing fewer deaths and hospitalizations than their older cousins — a sign that growing population immunity is tempering the immediate consequences of COVID-19 surges. Nature explores what the rise of BA.4 and BA.5 means for the pandemic. What are BA.4 and BA.5? The two variants are more similar to BA.2 than to the BA.1 strain that kicked off most countries’ Omicron waves late last year. But BA.4 and BA.5 carry their own unique mutations, including changes called L452R and F486V in the viral spike protein that might tweak its ability to latch onto host cells and skirt some immune responses. A May preprint1 found that BA.4 and BA.5 share an origin with earlier Omicron strains. But an unpublished analysis led by evolutionary geneticists Bette Korber and William Fischer at Los Alamos National Laboratory in New Mexico suggests that the variants are probably offshoots of BA.2 instead. Korber and Fischer also found that many genome sequences that are classified as BA.2 in public databases are actually BA.4 or BA.5. As a result, researchers could be underestimating the variants’ ongoing rise, as well as the diversity of mutations carried by them. “It is important in this particular moment in the pandemic to get these calls right,” Korber and Fischer wrote in an e-mail to Nature. Why are the variants on the rise globally? Variants’ transmission advantages can result from biological changes that speed infection, for instance, allowing the virus to infect more people, more quickly. But the rise of BA.4 and BA.5 seems to stem, instead, from their capacity to infect people who were immune to earlier forms of Omicron and other variants, says Christian Althaus, a computational epidemiologist at the University of Bern. With most of the world outside Asia doing little to control SARS-CoV-2, the rise — and inevitable fall — of BA.4 and BA.5 will be driven almost entirely by population immunity, Althaus adds, with cases increasing when protection lulls and falling only when enough people have been infected. On the basis of the rise of BA.5 in Switzerland — where BA.4 prevalence is low — Althaus estimates that about 15% of people there will get infected. But countries are now likely to have distinct immune profiles because their histories of COVID-19 waves and vaccination rates differ, Althaus adds. As a result, the sizes of BA.4 and BA.5 waves will vary from place to place. “It might be 5% in some countries and 30% in others. It all depends on their immunity profile,” he says. What impact will BA.4 and BA.5 have on society? This, too, is likely to vary by country. Despite high case numbers, South Africa experienced only a small rise in hospitalizations and deaths during its BA.4 and BA.5 wave, says Waasila Jassat, a public-health specialist at the country’s National Institute for Communicable Diseases in Johannesburg. In a study that will soon be posted to the medRxiv preprint server, Jassat and her colleagues found that South Africa’s BA.4 and BA.5 wave led to similar rate of hospitalization but slightly lower death rate when compared with the country’s earlier Omicron wave. Both Omicron surges proved much milder, in terms of hospitalizations and deaths, than the country’s ferocious Delta wave. Outside South Africa, other countries are seeing more significant impacts from BA.4 and BA.5. In Portugal — where COVID-19 vaccination and boosting rates are very high — the levels of death and hospitalization associated with the latest wave are similar to those in the first Omicron wave (although still nothing like the impact caused by earlier variants). One explanation for the difference could be Portugal’s demographics, says Althaus. “The more elderly people you have, the more severe disease.” Jassat thinks that the nature of a country’s immunity can also explain varying outcomes. About half of adult South Africans have been vaccinated, and just 5% have taken up a booster. But this, combined with sky-high infection rates from earlier COVID-19 waves, has erected a wall of ‘hybrid immunity’ that offers strong protection against severe disease, particularly in older people, who are the most likely to have been vaccinated, she adds. How well do vaccines work against the variants? Lab studies consistently suggest that antibodies triggered by vaccination are less effective at blocking BA.4 and BA.5 than they are at blocking earlier Omicron strains, including BA.1 and BA.22–6. This could leave even vaccinated and boosted people vulnerable to multiple Omicron infections, scientists say. Even people with hybrid immunity, stemming from vaccination and previous infection with Omicron BA.1, produce antibodies that struggle to incapacitate BA.4 and BA.5. Research teams have attributed that to the variants’ L452R and F486V spike mutations. One explanation for this is the observation that BA.1 infection after vaccination seems to trigger infection-blocking ‘neutralizing’ antibodies that recognize the ancestral strain of SARS-CoV-2 (the one that vaccines are based on) better than they recognize Omicron variants2,7. “Infection with BA.1 does induce a neutralizing antibody response, but it appears to be a little bit narrower than one would expect,” leaving people susceptible to immune-escaping variants such BA.4 and BA.5, says Ravindra Gupta, a virologist at the University of Cambridge, UK. What will come next? That’s anybody’s guess. The parade of Omicron subvariants could continue, with new variants picking further holes in existing immunity. “Nobody can say BA.4/5 is the final variant. It is highly probable that additional Omicron variants will emerge,” says Kei Sato, a virologist at the University of Tokyo. Researchers have identified several spots on the spike protein that are currently recognized by the antibodies that are triggered by vaccination and previous infection, but that could mutate in future Omicron strains2. Another possibility is the emergence of a variant from a branch of the SARS-CoV-2 family tree different from the one that bore Omicron. Repeat Omicron infections could build broad immunity against successive lineages, creating an opening for a totally different SARS-CoV-2 variant that is unfamiliar to people’s immune responses, says Gupta. “The bar is getting higher and higher for a virus to take over.” Increasingly, scientists think that variants including Omicron and Alpha probably originated from months-long chronic SARS-CoV-2 infections, in which sets of immune-evading and transmissibility-boosting mutations can build up. But the longer Omicron and its offshoots continue to dominate, the less likely it is that a totally new variant will emerge from a chronic infection, says Mahan Ghafari, who researches viral evolution at the University of Oxford, UK. To succeed, future variants will have to evade immunity. But they could come with other worrying properties. Sato’s team found that BA.4 and BA.5 were deadlier in hamsters than was BA.2, and better able to infect cultured lung cells6. Epidemiology studies, such as the one led by Jassat, suggest that successive COVID-19 waves are getting milder. But this trend should not be taken for granted, Sato cautions. Viruses don’t necessarily evolve to become less deadly. It’s also unclear when the next variant will appear. BA.4 and BA.5 started emerging in South Africa only a few months after BA.1 and BA.2, a pattern now being repeated in places including the United Kingdom and United States. But as global immunity from repeated vaccination and infection builds, Althaus expects the frequency of SARS-CoV-2 waves to slow down. One possible future for SARS-CoV-2 is that it will become like the other four seasonal coronaviruses, the levels of which ebb and flow with the seasons, usually peaking in winter and typically reinfecting people every three years or so, Althaus says. “The big question is whether symptoms will become milder and milder and whether issues with long COVID will slowly disappear,” he says. “If it stays like it is now, then it will be a serious public-health problem.” Published in Nature (June 23, 2022): https://doi.org/10.1038/d41586-022-01730-y

|
Scooped by
Juan Lama
|
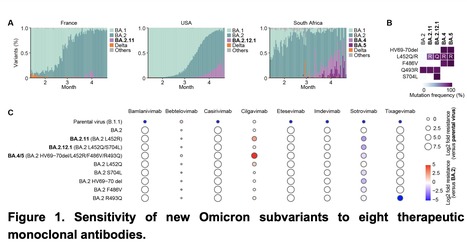
As of May 2022, Omicron BA.2 variant is the most dominant variant in the world. Thereafter, Omicron subvariants have emerged and some of them began outcompeting BA.2 in multiple countries. For instance, Omicron BA.2.11, BA.2.12.1 and BA.4/5 subvariants are becoming dominant in France, the USA and South Africa, respectively. In this study, we evaluated the sensitivity of these new Omicron subvariants (BA.2.11, BA.2.12.1 and BA.4/5) to eight therapeutic monoclonal antibodies (bamlanivimab, bebtelovimab, casirivimab, cilgavimab, etesevimab, imdevimab, sotrovimab and tixagevimab). Notably, we showed that although cilgavimab is antiviral against BA.2, BA.4/5 exhibits higher resistance to this antibody compared to BA.2. Since mutations are accumulated in the spike proteins of newly emerging SARS-CoV-2 variants, we suggest the importance of rapid evaluation of the efficiency of therapeutic monoclonal antibodies against novel SARS-CoV-2 variants. Preprint available at bioRxiv (May 3, 2022): https://doi.org/10.1101/2022.05.03.490409

|
Scooped by
Juan Lama
|
Two new sublineages of the Omicron coronavirus variant can dodge antibodies from earlier infection well enough to trigger a new wave, but are far less able to thrive in the blood of people vaccinated against COVID-19, South African scientists have found. The scientists from multiple institutions were examining Omicron's BA.4 and BA.5 sublineages - which the World Health Organization last month added to its monitoring list. They took blood samples from 39 participants previously infected by Omicron when it first showed up at the end of last year. Fifteen were vaccinated - eight with Pfizer's shot; seven with J&J's -- while the other 24 were not. "The vaccinated group showed about a 5-fold higher neutralisation capacity ... and should be better protected," said the study, a pre-print of which was released over the weekend. In the unvaccinated samples, there was an almost eightfold decrease in antibody production when exposed to BA.4 and BA.5, compared with the original BA.1 Omicron lineage. Blood from the vaccinated people showed a threefold decrease. South Africa may be entering a fifth COVID wave earlier than expected, officials and scientists said on Friday, blaming a sustained rise in infections that seems to be driven by the BA.4 and BA.5 Omicron sub-variants. Only about 30% of South Africa's population of 60 million is fully vaccinated. "Based on neutralisation escape, BA.4 and BA.5 have potential to result in a new infection wave," the study said. Cited research availanble (April 29, 2022) at https://secureservercdn.net/166.62.108.196/1mx.c5c.myftpupload.com/wp-content/uploads/2022/04/MEDRXIV-2022-274477v1-Sigal.pdf
|



 Your new post is loading...
Your new post is loading...