BioNTech is taking its mRNA mpox vaccine into the clinic, thanks in part to $90 million from a leading pandemic prevention organization. | BioNTech has partnered up with pandemic prevention organization CEPI to the tune of $90 million to help advance a couple of phase 1 stage mpox vaccines. The funding is part of CEPI's latest 100 Days Mission to help stymie future outbreaks. BioNTech began work on its vaccine, BNT166, as mpox (formerly known as monkeypox) began to spread globally in the summer of 2022. A phase 1/2 trial just got off the ground in August and will test two similar candidates (BNT166a and BNT166c) in patients with or without prior history of mpox infection. “Our work on mpox could broaden the portfolio of vaccines available against this potentially deadly disease, while building our understanding of how mRNA technology performs against orthopoxviruses, a family of viruses that have long afflicted humankind and remain an ongoing threat today,” CEPI CEO Richard Hatchett, M.D., said in the release....
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account

 Your new post is loading... Your new post is loading...
Neutralizing antibodies that target the BA.4 and BA.5 subvariants were four-fold higher in people aged 55 and older who received the bivalent booster than in those who received a monovalent booster. New data from Pfizer and BioNTech on their bivalent Covid-19 vaccine suggests the updated product may be more protective against more recent Omicron subvariants than the original version of the vaccine, the companies said in a statement released Friday. The companies said the levels of neutralizing antibodies that target the BA.4 and BA.5 subvariants of the SARS-CoV-2 virus were four-fold higher in people aged 55 and older who received the bivalent booster than in similarly aged people who received a monovalent booster. The bivalent, which was given an emergency use authorization at the end of August, targets both the original version of the SARS-2 virus and the BA.4/BA.5 variants. Recently BA.5 has been the dominant strain in the United States, but an alphabet soup of newer subvariants — BA.4.6, BQ.1.1 among them — is starting to crowd it out. The new data from the companies only looks at what getting the the booster did to antibody levels in recipients. The trial did not test whether people who received the updated boosters were less likely to contract Covid than people who received one of the older boosters.
“These data demonstrate that our BA.4/BA.5-adapted bivalent vaccine works as conceptually planned in providing stronger protection against the Omicron BA.4 and BA.5 sublineages,” Ugur Sahin, CEO and co-founder of BioNTech, said in the statement. “In the next step and as part of our science-based approach, we will continue to evaluate the cross-neutralization of the adapted vaccine against new variants and sublineages. Our aim is to provide broader immunity against Covid-19 caused by SARS-CoV-2, including Omicron and other circulating strains.” The companies also reported that one month after the trial participants got a dose of the bivalent booster, neutralizing antibodies targeting Omicron BA.4/BA.5 viruses increased 13.2-fold from pre-booster levels in adults who were older than 55 years of age; they increased 9.5-fold for adults 18 to 55 years of age. By comparison, in adults older than 55 who received a booster dose of the original vaccine, antibody titers to BA.4 and BA.5 rose 2.9-fold over the same period. Of late there have been a number of small studies that have tried to get an answer to the question of whether the updated vaccines are likely to be more protective than the original version against Omicron viruses. Three concluded that updating the vaccine did not make a difference while two suggested there was a benefit. But differences in the designs of the studies make them hard to compare to each other and to the Pfizer data. And at the end of the day, the important question is whether what was seen in terms of antibody production will translate into better protection for people who receive the bivalent vaccine, said Florian Krammer, a vaccinologist at Mount Sinai School of Medicine in Manhattan. He thought it might.
Pfizer press release (Nov. 04, 2022) available here:
The SARS-CoV-2 Omicron variant and its numerous sub-lineages have exhibited a striking ability to evade humoral immune responses induced by prior vaccination or infection. The Food and Drug Administration (FDA) has recently granted Emergency Use Authorizations (EUAs) to new bivalent formulations of the original Moderna and Pfizer mRNA SARS-CoV-2 vaccines that target both the ancestral strain as well as the Omicron BA.4/BA.5 variant. Despite their widespread use as a vaccine boost, little is known about the antibody responses induced in humans. Here, we collected sera from several clinical cohorts: individuals after three or four doses of the original monovalent mRNA vaccines, individuals receiving the new bivalent vaccines as a fourth dose, and individuals with BA.4/BA.5 breakthrough infection following mRNA vaccination.
Using pseudovirus neutralization assays, these sera were tested for neutralization against an ancestral SARS-CoV-2 strain, several Omicron sub-lineages, and several related sarbecoviruses. At ~3-5 weeks post booster shot, individuals who received a fourth vaccine dose with a bivalent mRNA vaccine targeting BA.4/BA.5 had similar neutralizing antibody titers as those receiving a fourth monovalent mRNA vaccine against all SARS-CoV-2 variants tested, including BA.4/BA.5. Those who received a fourth monovalent vaccine dose had a slightly higher neutralizing antibody titers than those who received the bivalent vaccine against three related sarbecoviruses: SARS-CoV, GD-Pangolin, and WIV1. When given as a fourth dose, a bivalent mRNA vaccine targeting Omicron BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not induce superior neutralizing antibody responses in humans, at the time period tested, compared to the original monovalent vaccine formulation.
Preprint available in bioRxiv (Oct. 24, 2022):
As Pfizer and Moderna's COVID-19 vaccine applications in young children move through the FDA's regulatory process, parents may soon be able to line their children up for shots. | As Pfizer and Moderna's COVID-19 vaccine applications in young children move through the FDA's regulatory process, parents may soon be able to line their children up for shots. At an advisory committee this week, independent experts will vote on whether to endorse Moderna’s vaccine in a wide range of children and adolescents and Pfizer’s in children 6 months through 4 years old. Ahead of the meeting, the FDA staffers posted their own findings, concluding that the vaccines are generally safe and effective in the respective age groups. Data on Pfizer's program showed that the three-dose series in children 6 months through 23 months of age was 75.6% effective, the reviewers said. Efficacy for the 2- to 4-years-old age group was 82.4%. The main adverse reactions in the 6-23 months group was tenderness at the injection site, irritability, drowsiness, decreased appetite and fever. Rates of adverse reactions for those recipients were lower than those in the 5- to 11-years-old age group, the FDA staff said. In the 2- to 4-year-old age group, adverse reactions included pain at the injection site, fatigue, headache and chills. Again, the rates were lower than those in the 5- to 11-years old group. In response to the FDA’s request for additional data on the vaccine's effectiveness against the delta and omicron variants, an analysis found the vaccine elicits a similar level of antibodies against delta and the reference strain, but noticeably lower levels against omicron.
As for Moderna's program, the vaccine is not yet authorized for adolescents, so Moderna is seeking a nod in children 6 months to 17 years old in two separate applications—one for children 6 months to 5 years and another in children 6 years to 17-years-old. Trials showed that vaccine efficacy for recipients 12- to 17-years-old was 93.3%, which is largely consistent with the efficacy in the adult study. The 6- to 11-years-old group saw an efficacy rate of 76.8%. The Moderna studies were conducted during the omicron surge and efficacy for this variant appeared consistent with efficacy observed among adults during the surge, the FDA staffers said. Meanwhile, efficacy in the 2- to 5-years-old group was lower at 36.8% using the CDC case definition. Finally in the 6 months through 23 months group, efficacy under the CDC definition was 50.6%. The most common reaction after receiving a dose was injection site pain, followed by headaches and fatigue. Other adverse reactions matched the placebo dose. Irritability or crying was another frequently reported and persistent reaction. COVID-19 doesn’t show signs of stopping with over 533 million cases and 6.3 million deaths worldwide, according to the CDC. If the FDA authorizes the children’s vaccines, shots could start going in arms by June 21, the Biden administration has said.
Pfizer and BioNTech have begun a clinical trial for their Omicron-specific Covid-19 vaccine candidate, they announced in a news release on Tuesday. The study will evaluate the vaccine for safety, tolerability and the level of immune response, as both a primary series and a booster dose, in up to 1,420 healthy adults ages 18 to 55.
The study is broken up into three groups: Participants in the first cohort have received two doses of the current Pfizer Covid-19 vaccine at least 90 to 180 days before the study. They will receive one or two doses of the Omicron-specific vaccine. Participants in the second cohort have received three doses of the current Pfizer Covid-19 vaccine at least 90 to 180 days prior to the study. They will receive one dose of the current Pfizer Covid-19 vaccine or the Omicron-specific vaccine. Participants in the third cohort have not received any Covid-19 vaccine. They will receive three doses of the Omicron-specific vaccine. The Omicron-specific vaccine will be administered as a 30-microgram dose, the same as the current vaccine. "While current research and real-world data show that boosters continue to provide a high level of protection against severe disease and hospitalization with Omicron, we recognize the need to be prepared in the event this protection wanes over time and to potentially help address Omicron and new variants in the future," Pfizer Senior Vice President and Head of Vaccine Research and Development Kathrin Jansen said in the release. Pfizer CEO Albert Bourla said last month that if a new vaccine is needed for the Omicron coronavirus variant, the company will have one in March. However, a Pfizer spokesperson confirmed that the company has already begun to manufacture this vaccine. "In the wake of Omicron, we are proactively investigating and manufacturing at risk an Omicron-based vaccine should it be needed, but we of course need to have results and discussions with health authorities as well as approvals before it would be deployed," the spokesperson told CNN. Expected vaccine production will not be affected if the companies need to pivot to the new vaccine, they said. "The companies have previously announced that they expect to produce four billion doses of the Pfizer-BioNTech COVID-19 Vaccine in 2022, and this capacity is not expected to change if an adapted vaccine is required." However, the companies also emphasized that people who have received booster doses of the current vaccine "maintain a high level of protection against Omicron, particularly against severe disease and hospitalizations." A new preprint lab study suggests that antibodies against the Omicron coronavirus variant remain robust four months after a third dose of the Pfizer/BioNTech vaccine. "Additional real world effectiveness data and laboratory investigations will further inform the duration of protection, potential need for an additional dose at a later time, and whether an Omicron modified vaccine is required," said the study from researchers at the University of Texas Medical Branch, Pfizer and BioNTech. Pfizer Press Release (Jan. 25, 2022):
Couple who pioneered jab given to millions around world tell how pharmaceutical giant wrongly assumed outbreak would be quickly contained. Pfizer initially turned down the offer of developing a coronavirus vaccine because its executives thought the virus would be rapidly contained. Dr Ugur Sahin and his wife, Dr Özlem Türeci, the founders of BioNTech, were told "guys, this is not going to work” by the pharmaceutical giant as the virus was starting to sweep the globe in January 2020. The mRNA technology, which has proved so crucial to the vaccine breakthroughs, was, at the time, also considered too experimental by Dr Phil Dormitzer, Pfizer’s vice-president and chief scientific officer for viral vaccines. “My working assumption was that it [Covid-19] would be controlled” like the Sars and Mers outbreaks, Dr Dormitzer admits. The initial rejection, revealed in a new book, came just days after the Turkish-born couple decided to dedicate BionNTech to creating an mRNA based Covid jab, effectively gambling the business on something that had never been done before. Their company is now worth US$85 billion. Yet Drs Sahin and Türeci remain close to Pfizer and Dr Dormitzer, or “Phil” as they know him. Dr Sahin had a detailed image in his mind of how the pandemic would unfold but also thought the Pfizer man’s assessment “completely rational”. “After the phone call with Phil, I just thought for a second and said ‘we will call him again in a few weeks,’” Dr Sahin told The Telegraph. The couple thought it only a “matter of time” before the drugs giant changed its mind – and they were right. A deal was announced between the two companies a month later. Next week, the Government is expected to announce a "booster" campaign for higher risk groups, which the Pfizer jab is expected to be at the forefront of. Boris Johnson will also announce the repeal of a series of measures from the Coronavirus Act which are now deemed unnecessary. These include powers to close-down sectors of the economy, such as business premises, or apply restrictions to events and gatherings. Husband and wife scientists’ vaccine gamble was a shot in the arm for millions
BioNTech may have created the world’s leading coronavirus vaccine but most of its staff are still working remotely, whether double or triple jabbed – and that includes its founders. “I’m here most of the time but the company is about 250 metres from here, so sometimes I'm just watching to see if everyone is working”, says Dr Ugur Şahin over a Zoom link from a temporary location. “It’s to avoid too much contact”, interjects his wife and business partner, Dr Özlem Türeci, perhaps sensing Dr Sahin’s joke with British journalists may go horribly wrong. “To let the people on the campus do their work in the labs and the manufacturing”. This dynamic - Dr Sahin enthusing, Dr Türeci copper-bottoming - may help explain how an obscure biotech firm from an unfashionable part of Germany ended up vaccinating so much of the world. So far, roughly 1.4 billion doses of their revolutionary jab - each containing billions of nanoparticles of synthetic RNA code - have been shipped to more than 120 countries. Only the Chinese vaccine manufacturers have distributed more. Their story is recounted in a new book, The Vaccine, written by journalist Joe Miller with the couples’ cooperation, to be released this week. It tells how the couple, who emigrated to Germany from Turkey as small children and met on a cancer ward as young doctors, built not one billion-dollar biotech company but two. And how they gambled everything to pivot BioNTech to focus exclusively on a Covid vaccine in early 2020. It’s proved a phenomenal success but when Dr Sahin initially called Pfizer to see if it wanted to be involved, the answer was a firm “no”.
“Guys, this is not going to work”, he was told by Dr Phil Dormitzer, Pfizer’s vice-president and chief scientific officer for vaccines. Mr Dormitzer had been involved with discussions about whether to create vaccines for Mers and Sars, only to see the pathogens quickly contained, and thought the same would be true of Sars-Cov-2. “My working assumption was that it would be controlled”, he later confirmed to Miller. None of this is recounted by the scientists with any hint of ego or malice. Dr Sahin is 56 but he exudes the energy of a 18 year old and does not seem to have a corporate bone in his body. He talks to us in a scruffy T-shirt with a hippy-ish thong around his neck. Dr Türeci is slightly more formal and has a doctor’s air of benevolent patience. “After the phone call with Phil, I just thought for a second and said ‘we will call him again in a few weeks,’” Dr Sahin recalls. It was the publication of a single article by Chinese academics in the Lancet on January 24 2020 that convinced the couple that a pandemic was coming and that they should act, no matter what the corporate risk. The article provided the first strong evidence of human-to-human transmission but, for Dr Sahin, there was something more: a seven-year-old girl mentioned in the study had tested positive for the virus without first displaying symptoms....
The decision will set off a cascade of vaccine requirements by hospitals, colleges, corporations, and some state and local governments. The Food and Drug Administration on Monday granted full approval to Pfizer-BioNTech’s coronavirus vaccine for people 16 and up, making it the first to move beyond emergency use status in the United States. The decision will set off a cascade of vaccine requirements by hospitals, colleges, corporations and other organizations. United Airlines recently announced that its employees will be required to show proof of vaccination within five weeks of regulatory approval. Oregon has adopted a similar requirement for all state workers, as have a host of universities in states from Louisiana to Minnesota. The Pentagon has said it would mandate the shots for the country’s 1.3 million active-duty troops once the Pfizer approval came through. The approval comes as the nation’s fight against the pandemic has intensified again, with the highly infectious Delta variant hurting the progress that the country had made over the first half of the year. The Biden administration hopes the development will motivate at least some of the roughly 85 million unvaccinated Americans who are eligible for shots to get them.
“While millions of people have already safely received Covid-19 vaccines, we recognize that for some, the F.D.A. approval of a vaccine may now instill additional confidence to get vaccinated,” Dr. Janet Woodcock, the acting F.D.A. commissioner, said in a statement. “Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.” Pfizer said it presented the F.D.A. with data from 44,000 clinical trial participants in United States, the European Union, Turkey, South Africa and South America. The company said the data showed the vaccine was 91 percent effective in preventing infection — a slight drop from the 95 percent efficacy rate that the data showed when the F.D.A. decided to authorize the vaccine for emergency use in December. Pfizer said the decrease reflected the fact that researchers had more time to catch people who became infected. A recent poll by the Kaiser Family Foundation, which has been tracking public attitudes during the pandemic, found that three of every 10 unvaccinated people said that they would be more likely to get vaccinated with a shot that had been fully approved. The Pfizer-BioNTech vaccine will continue to be authorized for emergency use for children ages 12 to 15 while Pfizer collects the necessary data required for full approval. A decision on whether to authorize the vaccine for children younger than 12 could be at least several months away. So far, more than 92 million Americans — 54 percent of those fully inoculated — have gotten Pfizer shots. Most of the rest received Moderna’s vaccine. Dr. Peter Marks, the F.D.A.’s top vaccine regulator, said that the Pfizer vaccine’s licensure followed a rigorous review of hundreds of thousands of pages of data and included inspections of the factories where the vaccine is produced. “The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.,” he said.
The F.D.A. is in the midst of a decision-making marathon related to coronavirus vaccines. The next major one looming for regulators is whether or not to authorize booster shots. The Biden administration said last week that pending the agency’s clearance, it will offer third shots to adults who got the Pfizer and Moderna vaccines eight months after their second injection, starting Sept. 20. Federal health officials said that both Pfizer-BioNTech and Moderna’s vaccines, which rely on similar technology, wane in potency over time. That trend, they said, is converging with the rise of the particularly dangerous Delta variant, making those who completed their vaccinations at the start of the year increasingly vulnerable to infection. Some health experts have challenged the decision to recommend booster shots as premature, saying the data shows that the vaccines are holding up well against severe disease and hospitalization, including against the Delta variant. Boosters would only be warranted if the vaccines were failing to prevent hospitalizations with Covid-19, some of those experts have said. Regulators are still reviewing Moderna’s application for full approval of its vaccine. That decision could take several weeks. Johnson & Johnson is expected to apply soon for full approval. Sharon LaFraniere is an investigative reporter. She was part of a team that won a Pulitzer Prize in 2018 for national reporting on Donald Trump’s connections with Russia. @SharonLNYT
Noah Weiland is a reporter in the Washington bureau, covering health care. He was raised in East Lansing, Mich., and graduated from the University of Chicago. @noahweiland
Neutralising Antibody Activity Against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 VaccinationThe SARS-CoV-2 B.1.617.2 Variant of Concern (VOC), first detected in India, is now dominant in the UK, having rapidly displaced the B.1.1.7 strain that emerged in the UK with the second COVID-19 wave in late 2020. The efficacy of currently licensed COVID-19 vaccines against B.1.617.2 is unknown; although it possesses 12 mutations in its spike protein relative to the wildtype SARS-CoV-2 first detected in Wuhan, China, in December, 2019, B.1.617.2 lacks mutations at amino acid positions 501 or 484 in its ACE2 receptor-binding domain, commonly associated with VOCs (appendix p 2) or escape from neutralising antibodies (NAbs). To determine vaccine-induced NAb escape by B.1.617.2 and compare activity to previous strains with existing estimates for population-based vaccine efficacy, we carried out an initial analysis of the Legacy study, established in January, 2021, by University College London Hospital and the Francis Crick Institute in London, UK, to track serological responses to vaccination in prospectively recruited staff volunteers (appendix p 6). A detailed description of the methods, including the clinical cohort, virus culture conditions, genetic sequencing, and neutralisation assays, and the statistical analysis are available in the appendix (p 8). The Legacy study was approved by London Camden and Kings Cross Health Research Authority Research and Ethics committee (IRAS number 286469) and sponsored by University College London. Published in the Lancet (June 3, 2021):
Background The SARS-CoV-2 pandemic has led to the development of various vaccines. Real-life data on immune responses elicited in the most vulnerable group of vaccinees over 80 years old is still underrepresented despite the prioritization of the elderly in vaccination campaigns. Methods We conducted a cohort study with two age groups, young vaccinees below the age of 60 and elderly vaccinees over the age of 80, to compare their antibody responses to the first and second dose of the BNT162b2 COVID-19 vaccination. Results While the majority of participants in both groups produced specific IgG antibody titers against SARS-CoV-2 spike protein, titers were significantly lower in elderly participants. Although the increment of antibody levels after the second immunization was higher in elderly participants, the absolute mean titer of this group remained lower than the <60 group. After the second vaccination, 31.3 % of the elderly had no detectable neutralizing antibodies in contrast to the younger group, in which only 2.2% had no detectable neutralizing antibodies. Conclusion Our data showed differences between the antibody responses raised after the first and second BNT162b2 vaccination, in particular lower frequencies of neutralizing antibodies in the elderly group. This suggests that this population needs to be closely monitored and may require earlier revaccination or/and an increased vaccine dose to ensure stronger long lasting immunity and protection against infection.
Published in Clinical Infectious Diseases (April 27, 2021):
Vaccinating many people against SARS-CoV-2 could stall infection rates even among unvaccinated children in the same community. Last December, Israel launched one of the fastest vaccination schemes in the world, reaching 50% of the population in 9 weeks. But only people aged 16 and over were eligible for the jab. To test the ripple effects of widespread vaccination, Tal Patalon at Maccabi Healthcare Services in Tel Aviv-Yafo, Israel, Roy Kishony at the Technion — Israel Institute of Technology in Haifa and their colleagues analysed COVID-19 vaccinations and test results recorded between January and March 2021 for people in 223 Israeli communities (O. Milman et al. Preprint at medRxiv https://doi.org/f4d7; 2021). In each community, the authors examined the relationship between the vaccination rate in adults over three 3-week intervals and the rate of positive results for a COVID-19 test in children 35 days later. The authors found that, in the weeks after older people had received the Pfizer–BioNTech vaccine, the infection risk among children under 16 dropped proportionally to the percentage of adults who had been vaccinated. The authors warn that their results might be influenced by children who had previously been infected, even though the study included communities with low infection rates. The findings have not yet been peer reviewed.
Preprint available in medRxiv (March 31, 2021):
Clinical trial results of Pfizer/BioNTech's Covid-19 vaccine show it is 100% efficacious and well tolerated in youths 12-15. Pfizer/BioNTech plan to submit the data to the US Food and Drug Administration as soon as possible for expanded emergency use authorization of the two-dose vaccine. In a Phase 3 trial of 2,260 participants ages 12 to 15 in the US, the vaccine elicited strong antibody responses one month after the second dose -- exceeding those demonstrated in people ages 16 to 25 in previous trials, Pfizer reported. The vaccine is currently authorized in the US for emergency use in people 16 and older. Researchers observed 18 Covid-19 cases among the 1,129 participants who were given a placebo, and none among the 1,131 volunteers who got the vaccine. The data has yet to be peer reviewed. Pfizer/BioNTech added that the side effects seen in the young teens were similar to those seen among 16 to 25-year-olds. Common side effects include pain at the injection site, fatigue and fever. The participants will be monitored for protection and safety for two years after their second dose. Those comparisons to the older population are important, because researchers are building off of the knowledge they gained in the adult trials.
Researchers can define a number of antibodies that are a correlate of the protection seen in adults, and then look for that level of antibodies in pediatric participants to know that the vaccine is providing protection. That's why the Covid-19 vaccine trials in children and adolescents have generally required fewer volunteers than the adult trials. "We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15," said Pfizer CEO Albert Bourla. "We plan to submit these data to FDA as a proposed amendment to our Emergency Use Authorization in the coming weeks and to other regulators around the world, with the hope of starting to vaccinate this age group before the start of the next school year." Dr. Peter Hotez, co-director of the Center for Vaccine Development at Texas Children's Hospital, said on CNN's New Day Wednesday, said schools can open without vaccinating students, but vaccines will help. "I think it's likely a green light to move forward, to move down in terms of vaccinating adolescents 12 to 15," Hotez said, noting that the vaccine will still need to be evaluated for authorization in that age group. "The bottom line is that by the fall I think there's a good possibility we'll be vaccinating teenagers, 12 and up, and for middle schools, junior high schools, high schools, it's really good news in the United States for both teachers and staff. We'll have teachers and staff vaccinated, we'll have the students vaccinated in those middle schools and high schools." A return to the classroom isn't the only factor at play. Health experts have emphasized the importance of protecting as many people as possible through vaccination, as more infectious Covid-19 variants continue to spread throughout the nation.
"We all long for a normal life. This is especially true for our children," said BioNTech CEO Ugur Sahin. "The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the B.1.1.7 UK variant." Pfizer recently told CNN that the safety demonstrated in this adolescent trial helped the company make the decision to begin testing its vaccine in younger children. A separate Phase 1/2/3 study of the Pfizer/BioNTech vaccine in children ages 6 months to 11 years launched last week, when the first children ages 5 to 11 received a shot. Pfizer/BioNTech plans to begin dosing 2 to 5-year-olds next week and work its way down to participants ages 6 months to 2 years. The company aims to enroll 4,644 children in the trial and expects results by the end of 2021. Moderna is also testing its vaccine in adolescents and children, in two clinical trials of children ages 12 to 17 and those ages 6 months to 11 years. Experts anticipate Covid-19 vaccines won't be available for children 11 and younger in time for the upcoming school year. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, has said those younger children may have to wait until the first quarter of 2022. Dr. Buddy Creech, director of Vanderbilt University's Vaccine Research Program and an investigator in Moderna's pediatric trials, estimates a Covid-19 vaccine could be available for high-risk kids 12 and older by July or August, but likely won't be available for children 11 and younger until November or December, at the earliest.
The COVID-19 vaccine from Pfizer Inc and BioNTech SE was able to neutralize a new variant of the coronavirus spreading rapidly in Brazil, according to a laboratory study published in the New England Journal of Medicine on Monday. Blood taken from people who had been given the vaccine neutralized an engineered version of the virus that contained the same mutations carried on the spike portion of the highly contagious P.1 variant first identified in Brazil, the study conducted by scientists from the companies and the University of Texas Medical Branch found. The scientists said the neutralizing ability was roughly equivalent the vaccine’s effect on a previous less contagious version of the virus from last year. The spike, used by the virus to enter human cells, is the primary target of many COVID-19 vaccines.
In previously published studies, Pfizer had found that its vaccine neutralized other more contagious variants first identified in the United Kingdom and South Africa, although the South African variant may reduce protective antibodies elicited by the vaccine. Pfizer has said it believes its current vaccine is highly likely to still protect against the South African variant. However, the drugmaker is planning to test a third booster dose of their vaccine as well as a version retooled specifically to combat the variant in order to better understand the immune response.
Original Study Published in N.Eng. J. Medicine (March 8, 2021): https://doi.org/10.1056/NEJMc2102017
The researchers said the findings support policies delaying the second shot in efforts to stretch supplies and speed up immunizations.
The findings vindicate the U.K.’s controversial strategy of waiting 12 weeks between doses, which divided experts and irked manufacturers, who said the strategy exceeds the parameters of clinical trials used to test the vaccine. The approach was reluctantly endorsed by the World Health Organization, though it stopped short of the British approach with only a six week delay between doses recommended in “exceptional circumstances.” The U.S. has previously rejected proposals to stretch limited vaccine supplies by delaying the second dose, but officials are reportedly reconsidering the strategy amid rapidly spreading variants of the virus. Israel’s vaccination campaign is, by a significant margin, the world’s fastest. Nearly half of its population has been vaccinated with one shot and almost a third has been fully vaccinated with two. As officials begin to mull an exit from societal lockdown, Yuli Edelstein, the country’s health minister, described getting “getting vaccinated is a moral duty” and “part of our mutual responsibility.” Under proposed plans, those wishing to reenter society will likely have to carry a certificate of vaccination.
Findigs in The Lancet (Feb. 18, 2021): https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00448-7/fulltext |
On September 1, 2022, the Moderna and Pfizer–BioNTech bivalent vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) containing equal amounts of spike messenger RNA from the ancestral and omicron BA.4–BA.5 subvariants replaced their monovalent counterparts as booster doses for persons who are 12 years of age or older in the United States. We previously reported surveillance data from North Carolina on the effectiveness of these two bivalent boosters against coronavirus disease 2019 (Covid-19) during the first 3 months after deployment (September 1 to December 8, 2022); the BA.4–BA.5 subvariants were predominant during the first 2.5 months of this period.1 Here, we present two additional months of data that were obtained during a period when the omicron BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants had become predominant to show the durability of protection conferred by these two bivalent boosters against a wider range of clinical outcomes than were included in our previous report. The data sources and study design have been described previously,1-3 and updated information is provided in the Methods section of the Supplementary Appendix, available with the full text of this letter at NEJM.org. The current study used data regarding booster doses and clinical outcomes from September 1, 2022, to February 10, 2023, for all North Carolina residents who were 12 years of age or older. During this period, a total of 6,306,311 residents were eligible to receive bivalent boosters; of these residents, 1,279,802 received the injections. A total of 19,462 of the 154,581 SARS-CoV-2 infections, 253 of the 2208 Covid-19–related hospitalizations, and 79 of the 867 Covid-19–related deaths occurred after receipt of the bivalent booster (Table S1 in the Supplementary Appendix).
We considered four outcome measures: infection, severe infection resulting in hospitalization, severe infection resulting in hospitalization or death, and severe infection resulting in death. We fit the Cox regression model with a time-varying hazard ratio for severe infection and fit the proportional-rates model with a time-varying rate ratio for recurrent infection for each additional booster dose that was received (i.e., first booster vs. primary vaccination, second booster vs. first booster, or third booster vs. second booster); all measures were adjusted for the baseline characteristics shown in Table S1. We estimated the booster effectiveness on a particular day as 1 minus the hazard ratio or rate ratio on that day multiplied by 100%.
Effectiveness against severe infection resulting in hospitalization was slightly lower, and effectiveness against infection was much lower. The effectiveness against severe infection resulting in death was the highest despite uncertainty because of the small number of events. We also analyzed the data separately for participants who received bivalent boosters before November 1, 2022 (when the BA.4–BA.5 subvariants were predominant) and after November 1, 2022 (when the BQ.1–BQ.1.1 subvariants were more prevalent and then were gradually replaced by the XBB–XBB.1.5 subvariants). The results are shown in the right column of Figure 1 and in Tables S3 and S4. The effectiveness was broadly similar between the two booster cohorts. Finally, we performed subgroup analyses according to the participant’s age and previous infection status and according to the manufacturers of the bivalent vaccine and the previous vaccine. Effectiveness against infection was higher for the Moderna bivalent vaccine than for the Pfizer–BioNTech bivalent vaccine and higher among previously infected participants than among those with no previous infection (Fig. S1). The two types of bivalent boosters were associated with an additional reduction in the incidence of omicron infection among participants who had previously been vaccinated or boosted. Although the two bivalent vaccines were designed to target the BA.4–BA.5 subvariants, they were also associated with a lower risk of infection or severe infection with the BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants. The effectiveness was higher against hospitalization and death than against infection and waned gradually from its peak over time.
Published in NEJM (April 12, 2023):
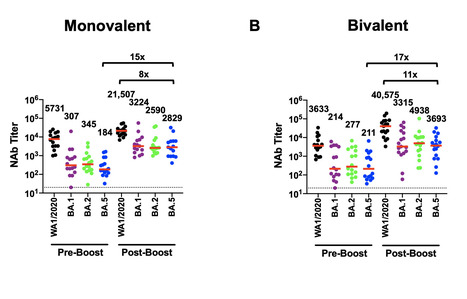
Waning immunity following mRNA vaccination and the emergence of SARS-CoV-2 variants has led to reduced mRNA vaccine efficacy against both symptomatic infection and severe disease. Bivalent mRNA boosters expressing the Omicron BA.5 and ancestral WA1/2020 Spike proteins have been developed and approved, because BA.5 is currently the dominant SARS-CoV-2 variant and substantially evades neutralizing antibodies (NAbs). Our data show that BA.5 NAb titers were comparable following monovalent and bivalent mRNA boosters.
Preprint available in bioRxiv:
Germany's BioNTech , Pfizer's partner in COVID vaccines, said the two companies would start tests on humans of next-generation shots that protect against a wide variety of coronaviruses in the second half of the year. Their experimental work on shots that go beyond the current approach include T-cell-enhancing shots, designed to primarily protect against severe disease if the virus becomes more dangerous, and pan-coronavirus shots that protect against the broader family of viruses and its mutations. In presentation slides posted on BioNTech's website for its investor day, the German biotech firm said its aim was to "provide durable variant protection". The two partners, makers of the Western world's most widely used COVID-19 shot, are currently discussing with regulators enhanced versions of their established shot to better protect against the Omicron variant and its sublineages. read more
The virus' persistent mutation into new variants that more easily evade vaccine protection, as well as waning human immune memory, have added urgency to the search by companies, governments and health bodies for more reliable tools of protection. As part of a push to further boost its infectious disease business, BioNTech said it was independently working on precision antibiotics that kill superbugs that have grown resistant to currently available anti-infectives. BioNTech, which did not say when trials could begin, is leaning on the technology of PhagoMed, which it acquired in October last year. The Vienna-based antibiotics developer has done work on enzymes, made by bacteria-killing viruses, that break through the bacterial cell wall. Drug-resistant infections are on the rise, driven by antibiotic overuse and leaks into the environment in antibiotics production. Public health researchers put the combined number of people dying per year from antibiotic-resistant infections in the United States and the European Union at close to 70,000. read more
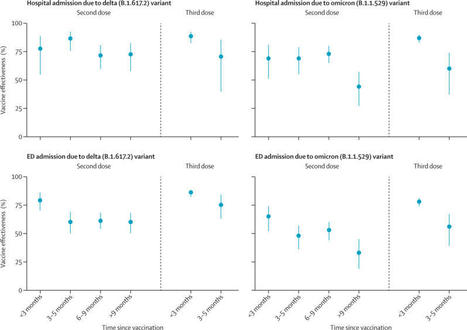
BackgroundThe duration of protection against the omicron (B.1.1.529) variant for current COVID-19 vaccines is not well characterised. Vaccine-specific estimates are especially needed. We aimed to evaluate the effectiveness and durability of two and three doses of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine against hospital and emergency department admissions due to the delta (B.1.617.2) and omicron variants. MethodsIn this case–control study with a test-negative design, we analysed electronic health records of members of Kaiser Permanente Southern California (KPSC), a large integrated health system in California, USA, from Dec 1, 2021, to Feb 6, 2022. Vaccine effectiveness was calculated in KPSC patients aged 18 years and older admitted to hospital or an emergency department (without a subsequent hospital admission) with a diagnosis of acute respiratory infection and tested for SARS-CoV-2 via PCR. Adjusted vaccine effectiveness was estimated with odds ratios from adjusted logistic regression models. This study is registered with ClinicalTrials.gov (NCT04848584). FindingsAnalyses were done for 11 123 hospital or emergency department admissions. In adjusted analyses, effectiveness of two doses of the BNT162b2 vaccine against the omicron variant was 41% (95% CI 21–55) against hospital admission and 31% (16–43) against emergency department admission at 9 months or longer after the second dose. After three doses, effectiveness of BNT162b2 against hospital admission due to the omicron variant was 85% (95% CI 80–89) at less than 3 months but fell to 55% (28–71) at 3 months or longer, although confidence intervals were wide for the latter estimate. Against emergency department admission, the effectiveness of three doses of BNT162b2 against the omicron variant was 77% (72–81) at less than 3 months but fell to 53% (36–66) at 3 months or longer. Trends in waning against SARS-CoV-2 outcomes due to the delta variant were generally similar, but with higher effectiveness estimates at each timepoint than those seen for the omicron variant. InterpretationThree doses of BNT162b2 conferred high protection against hospital and emergency department admission due to both the delta and omicron variants in the first 3 months after vaccination. However, 3 months after receipt of a third dose, waning was apparent against SARS-CoV-2 outcomes due to the omicron variant, including hospital admission. Additional doses of current, adapted, or novel COVD-19 vaccines might be needed to maintain high levels of protection against subsequent waves of SARS-CoV-2 caused by the omicron variant or future variants with similar escape potential. Published (April 22, 2022) in The Lancet Respiratory Medicine:
Vaccinated kids aged 5 to 11 showed evidence of protection against the virus, the company said. The data must be reviewed by the F.D.A. before children can be inoculated. The Pfizer-BioNTech coronavirus vaccine has been shown to be safe and highly effective in young children aged 5 to 11 years, the companies announced early Monday morning. The news sets the stage for authorization of the vaccine for younger children, possibly before the end of October. The need is urgent: Children now account for more than one in five new cases, and the highly contagious Delta variant has sent more children into hospitals and intensive care units in the past few weeks than at any other time in the pandemic. Pfizer and BioNTech plan to apply to the Food and Drug Administration by the end of September for authorization to use the vaccine in these children. If the regulatory review goes as smoothly as it did for older children and adults — it took roughly a month — millions of elementary school students could begin to receive shots around Halloween. Trial results for children younger than 5 are not expected till the fourth quarter of this year at the earliest, according to Dr. Bill Gruber, a senior vice president at Pfizer and a pediatrician. Results from Moderna’s vaccine trials in children under 12 are also expected around that time, said Dr. Paul Burton, the company’s chief medical officer. Pfizer and BioNTech announced the results in a statement that did not include detailed data from the trial. The findings have not yet been peer-reviewed nor published in a scientific journal. But the new results dovetail with those seen in older children and in adults, experts said. “There’s going to be a huge number of parents who are going to heave a big sigh of relief when they hear this,” said Dr. Kristin Oliver, a pediatrician and vaccine expert at Mount Sinai Hospital in New York. “We’ve been waiting for these kids to be protected.”
Before the vaccine can be authorized, F.D.A. scientists must carefully sift through the data, looking for side effects the company may have missed, which may slightly delay the process. Children have a much lower risk of Covid-19 than adults, even when exposed to the Delta variant. Still, some small number of infected children develop a life-threatening condition called multi-system inflammatory syndrome in children, or MIS-C. Still others may have lingering symptoms for months. Nearly 30,000 children were hospitalized for Covid in August; the least vaccinated states reported the highest rates. At Seattle Children’s hospital, about half of the children who are admitted for Covid are older than 12, according to Dr. Danielle Zerr, a pediatric infectious diseases expert at the hospital. “I’ve been dismayed at the fact that the sickest children in our hospital with acute Covid-19 or MIS-C are children who could have been vaccinated,” Dr. Zerr said. As ideological battles over masking and vaccine mandates play out in communities, the reopening of schools has fueled the surge. In Mississippi, among the states without a mask mandate, nearly 6,000 students tested positive for the virus in one week, and more than 30,000 students, teachers and staff had to be quarantined. One county in South Carolina — where mask mandates are banned — had to quarantine more than 2,000 students in one day. Remote learning is not an option in many districts, so the safety of some medically vulnerable children in many parts of the country has become subject to the actions of others. The trial results were greeted enthusiastically by many school administrators and teachers’ organizations, but are unlikely to lead to immediate policy changes. “This is one huge step toward beating Covid and returning to normalcy. I don’t think it changes the conversation around vaccine requirements for kids,” said Randi Weingarten, president of the American Federation of Teachers, a national union. Ms. Weingarten noted that parents and educators were still awaiting full F.D.A. approval of vaccines for children aged 12 to 15, and that mandates for adults did not come until months after the shots first became available....
Pfizer release (Sept. 20, 2021) available at:
BACKGROUNDPreapproval trials showed that messenger RNA (mRNA)–based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had a good safety profile, yet these trials were subject to size and patient-mix limitations. An evaluation of the safety of the BNT162b2 mRNA vaccine with respect to a broad range of potential adverse events is needed. METHODSWe used data from the largest health care organization in Israel to evaluate the safety of the BNT162b2 mRNA vaccine. For each potential adverse event, in a population of persons with no previous diagnosis of that event, we individually matched vaccinated persons to unvaccinated persons according to sociodemographic and clinical variables. Risk ratios and risk differences at 42 days after vaccination were derived with the use of the Kaplan–Meier estimator. To place these results in context, we performed a similar analysis involving SARS-CoV-2–infected persons matched to uninfected persons. The same adverse events were studied in the vaccination and SARS-CoV-2 infection analyses. RESULTSIn the vaccination analysis, the vaccinated and control groups each included a mean of 884,828 persons. Vaccination was most strongly associated with an elevated risk of myocarditis (risk ratio, 3.24; 95% confidence interval [CI], 1.55 to 12.44; risk difference, 2.7 events per 100,000 persons; 95% CI, 1.0 to 4.6), lymphadenopathy (risk ratio, 2.43; 95% CI, 2.05 to 2.78; risk difference, 78.4 events per 100,000 persons; 95% CI, 64.1 to 89.3), appendicitis (risk ratio, 1.40; 95% CI, 1.02 to 2.01; risk difference, 5.0 events per 100,000 persons; 95% CI, 0.3 to 9.9), and herpes zoster infection (risk ratio, 1.43; 95% CI, 1.20 to 1.73; risk difference, 15.8 events per 100,000 persons; 95% CI, 8.2 to 24.2). SARS-CoV-2 infection was associated with a substantially increased risk of myocarditis (risk ratio, 18.28; 95% CI, 3.95 to 25.12; risk difference, 11.0 events per 100,000 persons; 95% CI, 5.6 to 15.8) and of additional serious adverse events, including pericarditis, arrhythmia, deep-vein thrombosis, pulmonary embolism, myocardial infarction, intracranial hemorrhage, and thrombocytopenia. CONCLUSIONSIn this study in a nationwide mass vaccination setting, the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events examined. The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events was substantially increased after SARS-CoV-2 infection. (Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.)
Published in New England J. Medicine (August 25, 2021):
Scientists say mRNA technology could "open the floodgates" for developing vaccines against many other life-threatening conditions. BioNTech has announced plans to develop the world’s first mRNA-based malaria vaccine, in a potentially major step towards beating back the disease which still kills hundreds of thousands of people every year. The German pharmaceutical company produced its enormously successful Covid-19 vaccine along with the American giant Pfizer, and says it wants to start clinical trials of a shot to prevent malaria, made using similar technology, by 2022. Normal vaccines work by injecting a weakened or dead version of a germ into the human body to develop antibodies, but mRNA vaccines work differently. The new technology teaches cells how to make a protein that triggers an immune response, teaching the body to fight against future infections. "The very high efficacy of two mRNA vaccines for Covid-19 have shown the world just how powerful this technology could be against many diseases, including malaria," said Tedros Adhanom Ghebreyesus chief of World Health Organization, said last week.
Earlier this year, the team behind the Oxford-AstraZeneca vaccine developed the first effective jab against the mosquito-borne disease, with a more than 75 per cent efficacy rate. The hope is that vaccines like this could help stop the enormous and needless loss of life every year in Africa. Roughly 400,000 people die every year of the disease in sub-Saharan Africa, more than half of whom are under the age of five. Progress in tackling the ancient disease has recently stalled. Moreover, after a year in which African nations have struggled to acquire anywhere near enough Covid-19 vaccines to immunise their populations - all of which are manufactured overseas - BioNTech said it is looking for suitable vaccine production sites on the continent. BioNTech said the company would fund the research and initial production of the vaccine itself. Then it will turn to partners for support with large-scale trials. Scientists have said that mRNA technology could "open the floodgates" for developing vaccines against many other life-threatening conditions. BioNTech also plans to start a clinical trial to test a vaccine against tuberculosis in 2022. The organisation is also receiving support from the European Commission and the Bill and Melinda Gates Foundation and the World Health Organisation.
From
www
People who got mixed doses of coronavirus vaccines -- receiving a different vaccine type as a second dose than the first dose -- appear to be more likely to experience mild side effects such as fever, chills, fatigue or headache, researchers in the UK reported Wednesday. But the side effects following mix-and-match vaccinations were short-lived and there were no other safety concerns, the researchers reported in the Lancet medical journal. "These are the type of reactions you do expect with vaccine," Dr. Matthew Snape, an associate professor of pediatrics and vaccinology at the University of Oxford and chief investigator on the trial, said during a media briefing. "They are more or less the same types of reactions that you're seeing with the standard schedules. It's just that they're occurring more frequently, and we're seeing both more frequent both in mild and moderate symptoms -- but they resolved quickly," Snape said. Overall, "it's a really intriguing finding," he said, "and it's not something necessarily we were expecting -- to see such a consistent signal." It's something to keep an eye out for when giving mixed doses, the researchers said. "One of the things it's telling us is that, for example, you wouldn't want to immunize a ward full of nurses on the same day with a mixed schedule," Snape said. "Because you may have higher rates of absenteeism in the next day." The mix-and-match trialThe new research included 830 volunteers 50 and older who were randomly assigned to four different vaccine schedules involving the Oxford/AstraZeneca and Pfizer/BioNTech vaccines, with first and second doses given 28 days apart. They either got the AstraZeneca vaccine as both doses; AstraZeneca as a first dose and Pfizer as a second dose; the Pfizer vaccine as both doses; or the Pfizer vaccine as a first dose and AstraZeneca as a second dose. The researchers found that people who got different vaccines had more side effects following the second dose, with feverishness reported by 34% of those who received the AstraZeneca vaccine first and Pfizer vaccine second, compared with 10% of those given the AstraZeneca vaccine for both doses. Fever was reported by 41% of the people who received the Pfizer vaccine first and AstraZeneca vaccine second, compared with 21% of the volunteers given the Pfizer vaccine for both doses. "Similar increases were observed for chills, fatigue, headache, joint pain, malaise, and muscle ache," the researchers wrote. They noted that people could take acetaminophen -- sold under brand names such as Tylenol -- to ease the side-effects. There were no hospitalizations due to the symptoms and most of the increased reactions were seen within 48 hours after immunization, the researchers found. They noted that they did not see evidence of a rare blood clotting syndrome that's been linked with the AstraZeneca and Johnson & Johnson vaccines in any of the volunteers within a week after the second dose. The researchers also noted that their findings are based on initial data and there are now ongoing studies testing mixed administration of vaccines made by Moderna and Novavax. More research is also needed to evaluate immune responses following different types of schedules, and whether increased side effects suggest that schedules using different types of vaccines elicit strong immune responses. "We do think reactions often relate to the stimulating of the innate immune response," Snape said. "Whether or not this will relate to actually an improved immune response we don't know yet. We'll be finding out those results in a few weeks time. Seeking 'greater insight'The US Centers for Disease Control and Prevention and the World Health Organization do not currently recommend interchanging coronavirus vaccines -- but the CDC noted in January that its guidance may be updated as new information and new types of vaccines become available. There may be advantages to having more flexible vaccine schedules using different vaccine types as first and second doses, Dr. Jonathan Van-Tam, England's deputy chief medical officer, said in a statement in February when the new research first began. "Given the inevitable challenges of immunizing large numbers of the population against COVID-19 and potential global supply constraints, there are definite advantages to having data that could support a more flexible immunization program, if needed and if approved by the medicines regulator," Van-Tam said at the time. "It is also even possible that by combining vaccines, the immune response could be enhanced giving even higher antibody levels that last longer; unless this is evaluated in a clinical trial we just won't know," he said. "This study will give us greater insight into how we can use vaccines to stay on top of this nasty disease." See The Lancet publication (May 12):
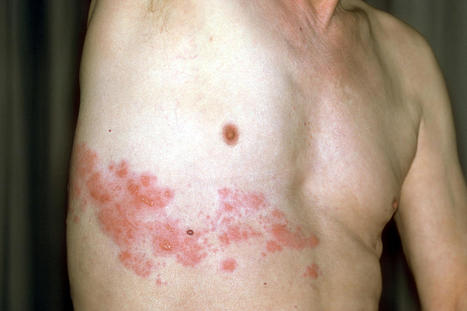
Herpes infections may be a side effect of the COVID-19 vaccine, experts have revealed. Scientists in Israel identified six cases in a new study of patients developing a skin rash known as herpes zoster after receiving the Pfizer vaccine, according to a study in the Rheumatology journal. Herpes zoster starts off as a small, itchy skin rash, but if left untreated, it could cause nerve damage and pain, the Jerusalem Post reported. This can include a prolonged burning sensation on the skin even after the rash disappears. Researchers from Tel Aviv Sourasky Medical Center and Carmel Medical Center in Haifa found those with autoimmune inflammatory rheumatic diseases had a higher risk of developing the herpes. Out of 491 patients, six people or 1.2 percent experienced the infection, researchers said. The six patients all have mild cases of autoimmune inflammatory rheumatic diseases and were young, though the infection is generally more common in those over the age of 50.
“That is why we reported on it,” Dr. Victoria Furer, the lead author, told the outlet. Five of them developed herpes zoster after the first dose and the sixth got it after the second. But it’s still unclear whether the vaccine caused the cases of herpes zoster. “We cannot say the vaccine is the cause at this point,” Furer told the outlet. “We can say it might be a trigger in some patients.” “We should not scare people,” she told the Jerusalem Post. “The overall message is to get vaccinated. It is just important to be aware.”
Original research published in Rheumathology (April 12, 2021):
Pfizer Inc and BioNTech said on Thursday their COVID-19 vaccine is around 91% effective at preventing the disease, citing updated trial data that included participants inoculated for up to six months. The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said.The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said. While the new overall efficacy rate of 91.3% is lower than the 95% originally reported in November for its 44,000-person trial, a number of variants have become more prevalent around the world since then. Pfizer’s Chief Executive Officer Albert Bourla said the updated result, which includes data on more than 12,000 people fully inoculated for at least six months, positions the drugmakers to submit for full U.S. regulatory approval. The vaccine is currently authorized on an emergency basis by the U.S. Food and Drug Administration.
The trial data “provide the first clinical results that a vaccine can effectively protect against currently circulating variants, a critical factor to reach herd immunity and end this pandemic for the global population,” Ugur Sahin, chief executive officer at BioNTech, said in a statement. Experts fear new variants of COVID-19 from South Africa and Brazil may be resistant to existing vaccines and treatment. More than 300 cases of the South African variant have been detected in more than 25 U.S. states and jurisdictions, according to federal data. Lab tests have previously indicated that BioNTech’s vaccine was less potent but still offered a robust defense against the B1351 variant that first emerged in South Africa. Still, BioNTech reiterated this week there would likely be a future need for booster shots that specifically address new variants and that the group was preparing to upgrade its vaccine when needed. BioNTech has said that it started testing a modified vaccine version against the South African mutant in March for early indications on safety and efficacy but a product for later market launch would require yet another redesign and more tests. The updated trial data would not prompt the company to change that development strategy, a BioNTech spokeswoman said.
Pfizer's Press Release (April 1, 2021):
COVID-19 vaccines from Moderna and Pfizer-BioNTech appear significantly less effective against the coronavirus variant first found in South Africa, a lab study has suggested. The percentage of protective antibodies that neutralized the variant — called B.1.351, which has been recorded in 20 US states — was 12.4 fold lower for Moderna's COVID-19 shot than against the original coronavirus, and 10.3 fold lower for Pfizer's, the study authors said. This was a bigger drop than in previous lab studies testing the vaccines against manufactured forms of the variant, they said. For this study, the researchers used real forms of the variant taken from people who had caught the virus. Both the Pfizer and Moderna vaccines have been authorized for emergency use in the US. B.1.351 was first detected in South Africa in October 2020. It has since spread to 42 countries, including to the US, where it is circulating in at least 20 states, including California and Texas, the Centers for Disease Control and Prevention said. There are 81 reported cases of B.1.351 in the US overall, the CDC said. The researchers found that the antibody activity from both vaccines was "essentially unchanged" against the variant first found in the UK, B.1.1.7. There are 3,037 reported cases in the US of B.1.1.7, the CDC said, and experts believe it will soon become the dominant strain in the US. The scientists, from Columbia University, also tested lab-made viruses that had certain mutations. They said that one specific mutation, E484K, appeared to be a "major contributor" to the B.1.351 variant's ability to evade the antibody response. E484K is not usually present in B.1.1.7, the variant first found in the UK. The study has been accepted by science journal Nature but not yet published.
Taking samples from the real worldIn the experiment, scientists took 10 blood samples from people who had received two doses of Pfizer's vaccine, 28 days after their second dose, and 12 samples from those who had received two doses of Moderna's vaccine, 43 days after their second dose. They then compared how well antibodies in the blood samples "neutralized" the original coronavirus, compared to real-life B.1.1.7 and B.1.351 coronavirus variants. The sample size was small, and the antibody response is just one aspect of the immune response, so it remains unclear how well the vaccines work against the variant first found in South Africa in real life. Pfizer has conducted petri-dish tests before that showed a less potent antibody response against a lab-made coronavirus variant that mimicked the variant first found in South Africa. It was not the exact B.1.351 variant. The study, published as correspondence to the New England Journal of Medicine February 17 and updated March 8, suggested the vaccine would work against the variant. It also showed that the antibody response from Pfizer's shot held up against the variant first found in Brazil, P.1, that has a similar set of mutations to B.1.351. Moderna ran similar tests and said that its vaccine held up well against the mutations found in B.1.1.7, the variant first found in the UK, but less well against the mutations found in B.1.351, the variant first identified in South Africa. Again, it used lab-made variants. Both companies said in January that they were developing booster shots specifically to tackle the B.1.351 variant. Neither of the vaccines has been properly tested against the variant first found in South Africa in the real world. In Israel, Pfizer's vaccine has been shown to be highly effective against the B.1.1.7 variant, first found in the UK. About 80% of Israelis with COVID-19 are infected with B.1.1.7. The COVID-19 vaccine from Johnson & Johnson was less effective in the clinical trials that took place in South Africa. Original Findings to Be Published in Nature (March 8, 2021):
Asymptomatic coronavirus infections were four times less frequent in health-care workers who had received a single dose of a prominent COVID-19 vaccine than in their unvaccinated counterparts. Michael Weekes at the University of Cambridge, UK, and his colleagues analysed the results of almost 8,900 SARS-CoV-2 tests taken by UK health-care workers without symptoms of COVID-19 (M. Weekes et al. Preprint at Authorea https://doi.org/fxkd; 2021). Study participants who were tested at least 12 days after receiving one dose of the vaccine developed by Pfizer of New York City and BioNTech of Mainz, Germany, had an infection rate of only 0.2%. By contrast, unvaccinated participants had an infection rate of 0.8%.
The team also noted that participants who showed evidence of SARS-CoV-2 infection well after vaccination tended to have lower levels of the coronavirus in their bodies than did those who were infected and unvaccinated, although the result did not reach statistical significance. If corroborated, this would suggest that the few vaccinated health-care workers who do have an asymptomatic infection are less likely to infect other people than are unvaccinated workers who become infected. The findings have not yet been peer reviewed. |