Your new post is loading...

|
Scooped by
Juan Lama
|
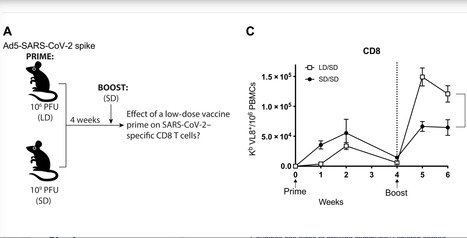
SARS-CoV-2 has caused a global pandemic that has infected more than 250 million people worldwide. Although several vaccine candidates have received emergency use authorization, there is still limited knowledge on how vaccine dosing affects immune responses. We performed mechanistic studies in mice to understand how the priming dose of an adenovirus-based SARS-CoV-2 vaccine affects long-term immunity to SARS-CoV-2. We first primed C57BL/6 mice with an adenovirus serotype 5 vaccine encoding the SARS-CoV-2 spike protein, similar to that used in the CanSino and Sputnik V vaccines. The vaccine prime was administered at either a standard dose or 1000-fold lower dose, followed by a boost with the standard dose 4 weeks later. Initially, the low dose prime induced lower immune responses relative to the standard dose prime. However, the low dose prime elicited immune responses that were qualitatively superior and, upon boosting, exhibited substantially more potent recall and functional capacity. We also report similar effects with a simian immunodeficiency virus (SIV) vaccine. These findings show an unexpected advantage of fractionating vaccine prime doses, warranting a reevaluation of vaccine trial protocols for SARS-CoV-2 and other pathogens. Published in Science Immunology: https://doi.org/10.1126/sciimmunol.abi8635

|
Scooped by
Juan Lama
|
High-profile COVID-19 vaccines developed in Russia and China share a potential shortcoming: They are based on a common cold virus that many people have been exposed to, potentially limiting their effectiveness, some experts say. CanSino Biologics’ vaccine, approved for military use in China, is a modified form of adenovirus type 5, or Ad5. The company is in talks to get emergency approval in several countries before completing large-scale trials, the Wall Street Journal reported last week. A vaccine developed by Moscow’s Gamaleya Institute, approved in Russia earlier this month despite limited testing, is based on Ad5 and a second less common adenovirus. “The Ad5 concerns me just because a lot of people have immunity,” said Anna Durbin, a vaccine researcher at Johns Hopkins University. “I’m not sure what their strategy is … maybe it won’t have 70 percent efficacy. It might have 40 percent efficacy, and that’s better than nothing, until something else comes along.” Vaccines are seen as essential to ending the pandemic that has claimed over 845,000 lives worldwide. Gamaleya has said its two-virus approach will address Ad5 immunity issues. Both developers have years of experience and approved Ebola vaccines based on Ad5. Neither CanSino nor Gamaleya responded to requests for comment. Researchers have experimented with Ad5-based vaccines against a variety of infections for decades, but none are widely used. They employ harmless viruses as “vectors” to ferry genes from the target virus — in this case the novel coronavirus — into human cells, prompting an immune response to fight the actual virus. But many people already have antibodies against Ad5, which could cause the immune system to attack the vector instead of responding to the coronavirus, making these vaccines less effective. Several researchers have chosen alternative adenoviruses or delivery mechanisms. Oxford University and AstraZeneca based their COVID-19 vaccine on a chimpanzee adenovirus, avoiding the Ad5 issue. Johnson & Johnson’s candidate uses Ad26, a comparatively rare strain. Dr. Zhou Xing, from Canada’s McMaster University, worked with CanSino on its first Ad5-based vaccine, for tuberculosis, in 2011. His team is developing an inhaled Ad5 COVID-19 vaccine, theorizing it could circumvent pre-existing immunity issues. “The Oxford vaccine candidate has quite an advantage” over the injected CanSino vaccine, he said. Xing also worries that high doses of the Ad5 vector in the CanSino vaccine could induce fever, fueling vaccine skepticism. “I think they will get good immunity in people that don’t have antibodies to the vaccine, but a lot of people do,” said Dr. Hildegund Ertl, director of the Wistar Institute Vaccine Center in Philadelphia. In China and the United States, about 40 percent of people have high levels of antibodies from prior Ad5 exposure. In Africa, it could be has high as 80 percent, experts said. Some scientists also worry an Ad5-based vaccine could increase chances of contracting HIV. In a 2004 trial of a Merck & Co Ad5-based HIV vaccine, people with pre-existing immunity became more, not less, susceptible to the virus that causes AIDS. Researchers, including top U.S. infectious diseases expert Dr. Anthony Fauci, in a 2015 paper, said the side effect was likely unique to HIV vaccines. But they cautioned that HIV incidence should be monitored during and after trials of all Ad5-based vaccines in at-risk populations. Gamaleya’s vaccine will be administered in two doses: The first based on Ad26, similar to J&J’s candidate, and the second on Ad5. Alexander Gintsburg, Gamaleya’s director, has said the two-vector approach addresses the immunity issue. Ertl said it might work well enough in individuals who have been exposed to one of the two adenoviruses.

|
Scooped by
Juan Lama
|
The vaccine generated an immune response in a study of roughly 1,000 patients, and all of the side effects were deemed mild or moderate. The data, published in the medical journal the Lancet, also show that the vaccine caused side effects, including fever, headaches, muscle aches, and injection site reactions, in about 60% of patients. All of the side effects were deemed mild or moderate, and all resolved themselves over the course of the study. While the Oxford-AstraZeneca vaccine, known as AZD1222, has moved most rapidly into larger-scale studies of any major contender — and AstraZeneca has said that billions of doses could be manufactured — the new data represent the first glimpse researchers have gotten at its efficacy. They show a relatively safe vaccine, though side effects were greater than for a meningitis vaccine, to which it was compared, that engages the immune system to fight the virus. The Lancet also published results of another vaccine, from the Chinese biotech CanSino, that had been previously released. The Phase 2 results showed that, as was seen in the Phase 1 data, the vaccine induced neutralizing antibody responses — which could be vital to preventing the disease’s dangerous symptoms — in most subject. But further study continues to show that this vaccine works better in some people than others. And among those it didn’t work as well in were people aged 55 and older, a key target for Covid-19 vaccination. “The results of both studies augur well for phase 3 trials, where the vaccines must be tested on much larger populations of participants to assess their efficacy and safety,” wrote two vaccine researchers from Johns Hopkins University, Naor Bar-Zeev and William Moss, in an accompanying editorial. The data on the Oxford-AstraZeneca vaccine do not provide enough information to predict whether it will be more effective than other vaccines that are also entering clinical trials. AZD1222 is one of 23 potential Covid-19 vaccines that are being tested in clinical trials, according to the World Health Organization. Studies in which thousands of volunteers are given either a vaccine or placebo are necessary to know for sure if any vaccine prevents infection with SARS-Cov-2, and how well that vaccine works. No such studies have been completed. AZD1222 is one of 23 potential Covid-19 vaccines that are being tested in clinical trials, according to the World Health Organization. Studies in which thousands of volunteers are given either a vaccine or placebo are necessary to know for sure if any vaccine prevents infection with SARS-Cov-2, and how well that vaccine works. No such studies have been completed. The vaccine also produced a response in T cells, a type of white blood cell that attacks cells infected with viruses, according to the paper. In a statement, Andrew Pollard of the University of Oxford, the study’s lead author, said that the vaccine is intended to induce both types of responses. “We hope this means the immune system will remember the virus, so that our vaccine will protect people for an extended period,” he said... Original study for the Oxford vaccine published at the Lancet (July 2020): https://doi.org/10.1016/S0140-6736(20)31604-4 Original study of Cansino's vaccine published at the Lancet (July 2020): https://doi.org/10.1016/S0140-6736(20)31605-6 See also Editorial comment (July 2020): https://doi.org/10.1016/S0140-6736(20)31611-1

|
Scooped by
Juan Lama
|
CanSino and Moderna are the first vaccine makers to report data on safety and neutralization, but the extent of protection these products afford remains unclear. CanSino Biologics and a team of researchers from seven different Chinese institutions have published the results of a phase 1 trial of the company’s SARS-CoV-2 spike (S) protein vaccine. The intramuscularly injected adenoviral vaccine elicited both neutralizing antibodies and a T-cell response in a phase 1 study in 108 volunteers. A week earlier, Moderna raised a cool $1.34 billion in a share offering after it press-released preliminary data from just eight healthy volunteers from a phase 1 trial of its mRNA vaccine candidate against SARS-CoV-2. The limited data released suggest that their vaccine can stimulate the production of neutralizing antibodies and appears to be safe in a tiny group of individuals; it is not clear how these relate to antibody responses in the general population. CanSino’s open-label dose-escalation study found that 75–83% of patients in three groups, each with a different dosage (5 × 1011, 1 × 1012 or 5 × 1012 viral particles), reported at least one adverse reaction by day 7, such as pain at the injection site and sometimes fever, fatigue, headache and muscle pain. The effects were most severe at the highest dose, and the vaccine’s developers plan to take the medium dose into a phase 2 trial. Tantalizingly, the preliminary data also indicate the adenoviral serotype 5 (Ad5) vaccine generates neutralizing antibody and T-cell responses, although whether the reported responses will prove to be protective is open to question. “We have no idea what titers are needed to protect,” says Hildegund Ertl, professor in the Wistar Institute’s Vaccine & Immunotherapy Center. The lack of a standardized virus neutralization assay for SARS-CoV-2 complicates data interpretation. “As long as people are using different assays, it’s going to be hard to figure that out.” But just 50% of those who received the medium dose developed neutralizing antibodies, a rate that, Ertl says, “is not particularly good.” The T-cell response was “not particularly impressive” either...

|
Scooped by
Juan Lama
|
Among a long list of biopharma companies developing vaccines against the novel coronavirus, China’s CanSino Biologics and Massachusetts-based Moderna Therapeutics are so far the front-runners. After Moderna dosed its first subject this week, CanSino said it's been cleared to start its trials. CanSino Bio and its collaborators at the Academy of Military Medical Sciences’ Institute of Biotechnology secured a quick Chinese regulatory go-ahead to start human testing of their recombinant coronavirus vaccine, the company said in a disclosure (PDF) to the Hong Kong Stock Exchange on Wednesday. “Thanks to our collaborators and our diligent team, who worked almost around the clock since late January to develop this vaccine candidate with sound scientific data to support IND filing,” CanSino Chairman and CEO Xuefeng Yu said in a statement. The green light was doled out very quickly. It was only Tuesday when CanSino said it had filed the pre-IND review application for the vaccine to authorities and was in the process of a rolling submission of technical documents. CanSino’s candidate, dubbed Ad5-nCoV, uses its adenovirus-based viral vector vaccine platform. In 2017, the same technology helped the company earn a Chinese nod for its Ebola vaccine, which was the first Ebola shot approved anywhere based on the strain behind the deadly epidemic in West Africa in 2014. The new phase 1 trial will be conducted in healthy adults 18 to 60 years of age in Wuhan, China, which was first to report cases of the current SARS-CoV-2 virus. Investigators plan to divide 108 participants into three groups to receive different doses of the vaccine, according to information posted on the Chinese Clinical Trial Registry. While the study’s main objective is to examine the vaccine’s safety, researchers will also evaluate efficacy measures, including levels of an antibody against the spike protein on the coronavirus cell surface that’s key for infection, as well as neutralizing antibody against SARS-CoV-2.
|

|
Scooped by
Juan Lama
|
Adenovirus vectors deliver the genetic instructions for SARS-CoV-2 antigens directly into patients' cells, provoking a robust immune response. But will pre-existing immunity from common colds take them down? Six vaccine candidates in clinical trials for COVID-19 employ viruses to deliver genetic cargo that, once inside our cells, instructs them to make SARS-CoV-2 protein. This stimulates an immune response that ideally would protect recipients from future encounters with the actual virus. Three candidates rely on weakened human adenoviruses to deliver the recipe for the spike protein of the pandemic coronavirus, while two use primate adenoviruses and one uses measles virus. Most viral vaccines are based on attenuated or inactivated viruses. An upside of using vectored vaccines is that they are easy and relatively cheap to make. The adenovirus vector, for example, can be grown up in cells and used for various vaccines. Once you make a viral vector, it is the same for all vaccines, says Florian Krammer, a vaccinologist at the Icahn School of Medicine at Mount Sinai. “It is just the genetic information in it that is different,” he explains. Once inside a cell, viral vectors hack into the same molecular system as SARS-CoV-2 and faithfully produce the spike protein in its three dimensions. This resembles a natural infection, which provokes a robust innate immune response, triggering inflammation and mustering B and T cells. But the major downside to the human adenoviruses is that they circulate widely, causing the common cold, and some people harbor antibodies that will target the vaccine, making it ineffective. CanSino reported on its Phase II trial this summer of its COVID-19 vaccine that uses adenovirus serotype 5 (Ad5). The company noted that 266 of the 508 participants given the shot had high pre-existing immunity to the Ad5 vector, and that older participants had a significantly lower immune response to the vaccine, suggesting that the vaccine will not work so well in them. “The problem with adenovirus vectors is that different populations will have different levels of immunity, and different age groups will have different levels of immunity,” says Nikolai Petrovsky, a vaccine researcher at Flinders University in Australia. Also, with age, a person accumulates immunity to more serotypes. “Being older is associated with more chance to acquire Ad5 immunity, so those vaccines will be an issue [with elderly people],” Krammer explains. Moreover, immunity against adenoviruses lasts for many years. “A lot of people have immunity to Ad5 and that impacts on how well the vaccine works,” says Krammer. In the US, around 40 percent of people have neutralizing antibodies to Ad5. As part of her work on an HIV vaccine, Hildegund Ertl of the Wistar Institute in Philadelphia previously collected serum in Africa to gauge resistance levels to this and other serotypes. She found a high prevalence of Ad5 antibodies in sub-Saharan Africa and some West African countries—80 to 90 percent. A different group in 2012 reported that for children in northeast China, around one-quarter had moderate levels and 9 percent had high levels of Ad5 antibodies. “I don’t think anyone has done an extensive enough study to do a world map [of seroprevalence],” notes Ertl. J&J’s Janssen is using a rarer adenovirus subtype, Ad26, in its COVID-19 vaccine, reporting in July that it protects macaques against SARS-CoV-2 and in September that it protects against severe clinical disease in hamsters. Ad26 neutralizing antibodies are uncommon in Europe and the US, with perhaps 10–20 percent of people harboring antibodies. They are more common elsewhere. “In sub-Saharan Africa, the rates are ranging from eighty to ninety percent,” says Ertl. Also critical is the level of antibodies in individuals, notes Dan Barouch, a vaccinologist at Beth Israel Deaconess Medical Center and Harvard Medical School. For instance, there was no neutralizing of Ad26-based HIV and Ebola vaccines in more than 80,000 people in sub-Saharan Africa, he says. “Ad26 vaccine responses do not appear to be suppressed by the baseline Ad26 antibodies found in these populations,” because the titres are low, Barouch writes in an email to The Scientist. Barouch has long experience with Ad26-based vaccines and collaborates with J&J on their COVID-19 vaccine. The Russian Sputnik V vaccine, approved despite no published data or Phase 3 trial results, starts with a shot of Ad26 vector followed by a booster with Ad5, both of which carry the gene for the spike protein of SARS-CoV-2. This circumvents a downside of viral vector vaccines, specifically, once you give the first shot, subsequent injections will be less efficacious because of antibodies against the vector. Ertl says she has no idea of the proportion of the Russian population with Ad26 or Ad5 antibodies, and there seems to be little or no published data from countries that have expressed interested in this virus, such as Venezuela and the Philippines...

|
Scooped by
Juan Lama
|
Through Operation Warp Speed, the U.S. has backed seven vaccine candidates with $11 billion, securing preorders for some 800 million doses. Scientists, drugmakers and governments are moving with unprecedented haste to deliver a vaccine to protect against the new coronavirus. The fastest of them have already delivered early data from human studies, and further results from others should come quickly as the year progresses. The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world. Researchers' success or failure could determine whether the virus becomes endemic, recurring in countries around the world year after year, or is ultimately checked. With the health of their citizens at stake, governments are investing enormous sums of money into vaccine research and development, and to prepare for manufacturing and distributing what will likely need to be hundreds of millions of doses necessary to keep infection at bay. With modern-day Manhattan Projects underway, vaccines have become an issue of national security, too, raising questions of global equity and medicine access. In the U.S., the Trump administration has unveiled "Operation Warp Speed," so far pledging more than $11 billion in funding and support for seven candidates. There's no guarantee the first successful vaccine will come from the U.S., however. Some of the leading candidates are being developed overseas, with projects by the University of Oxford in the U.K. and China's CanSino Biologics the furthest along. The rest of the world might not be so lucky. "It's not like we can expect 7 billion doses the day after licensure so we can vaccinate the whole world," said Emory University vaccines expert Walter Orenstein. Yet, to truly curb circulation of the SARS-CoV-2 virus in humans, getting vaccines to nations wealthy and poor will be a vital mission. The next few months should produce a flurry of data, early answers and fresh questions, making it difficult to keep track. Here's where things stand for 13 of the most advanced, most promising or best funded vaccine candidates in the pipeline.

|
Scooped by
Juan Lama
|
The Chinese government has approved the use of an experimental Covid-19 vaccine for the country's military -- the latest step in a global race to stop the deadly disease caused by the novel coronavirus. The vaccine, known as Ad5-nCoV, was jointly-developed by the Beijing Institute of Biotechnology -- part of the Chinese government's Academy of Military Medical Sciences -- and vaccine company CanSino Biologics. In a statement to the Hong Kong Stock Exchange on Monday, CanSino announced that China's Central Military Commission had given the vaccine a "military specially-needed drug approval" on June 25. The special permission will last for one year and will only apply to military personnel. China has repeatedly insisted that its military has remained unaffected by the pandemic, with officials claiming that the People's Liberation Army (PLA), has not recorded a single coronavirus case. US observers, however, have cast doubt on the claims, noting that the PLA is the among the world's largest standing armies, making it statistically unlikely that its personnel have not been exposed to the virus. Neither the Chinese government nor CanSino have said how broadly the vaccine will be distributed, which units were be selected or whether it will be mandatory or voluntary for personnel. CNN has reached out for CanSino for comment on the announcement. According to a CanSino statement, clinical trials of the new vaccine have shown a "good safety profile" with initial results indicating that Ad5-nCoV had potential to prevent diseases caused by SARS-CoV-2, the coronavirus strain that causes Covid-19....

|
Scooped by
Juan Lama
|
A Covid-19 vaccine candidate being developed by a Chinese drug maker appeared to induce an immune response in subjects, but also showed some concerning although not unexpected results. Data on the vaccine, made by CanSino Biologics, were published Friday in the Lancet, the first time Phase 1 trial data from any Covid-19 vaccine have been published in a scientific journal. The results are likely to be closely examined, particularly in Canada, which recently announced it would test the vaccine and produce it there if results of the early studies were positive. The study found that one dose of the vaccine, tested at three different levels, appeared to induce a good immune response in some subjects. But about half of the volunteers — people who already had immunity to the backbone of the vaccine — had a dampened immune response. The vaccine is what’s known as a viral vector vaccine; it uses a live but weakened human cold virus, adenovirus 5, onto which genetic material of the SARS-CoV-2 coronavirus has been fused. The Ad5 virus is effectively a delivery system that teaches the immune system to recognize the coronavirus. But many people have had previous infections with adenovirus 5, raising concerns that the immune system would focus on the Ad5 parts of the vaccine rather than the SARS-Cov-2 part. Many research groups that work on viral-vectored vaccines stopped using Ad5 because of concerns about preexisting immunity, which can run to 70% or higher in some populations. “This is definitely one of the concerns about using vectored vaccines for which people might already have pre-existing immunity,” said Michael Mina, an infectious diseases epidemiologist at Harvard’s T.H. Chan School of Public Health. “If you already have seen a virus or have some pre-existing immunity to it … you run the risk of having your immune response get skewed and picking up primarily the thing you’re already immune to or that you’ve already seen and not focusing so much on the new aspect, which in this case would be the coronavirus proteins that were placed onto the adenovirus vector,” he said. In the study, Chinese scientists reported that while people who had high levels of preexisting immunity to Ad5 responded to the vaccine, the rise in antibodies to the SARS-Cov-2 virus was less robust than among those in the study who had low or no preexisting antibodies to Ad5. They also showed antibodies to the adenovirus itself soared among people who had prior immunity, suggesting their systems views the vaccination as a boost of their Ad5 immunity..... Studiy Published in The Lancet (May 22, 2020): https://doi.org/10.1016/S0140-6736(20)31208-3
|



 Your new post is loading...
Your new post is loading...