Your new post is loading...

|
Scooped by
Juan Lama
|
Explore the latest developments in Multiple Sclerosis research, focusing on the potential of a vaccine targeting the Epstein-Barr Virus. Learn about the impact of EBV on MS and the groundbreaking efforts towards vaccine development. Recent scientific advancements have brought hope in the fight against Multiple Sclerosis (MS), a debilitating neurological condition affecting millions worldwide. Key to this optimism is the potential development of a vaccine targeting the Epstein-Barr Virus (EBV), a common factor in nearly all MS patients. Understanding Multiple Sclerosis and EBV Multiple Sclerosis is an autoimmune disease that leads to the destruction of myelin, the protective sheath surrounding nerve fibers. This results in a wide range of symptoms, including severe physical disability. The exact cause of MS remains unknown, but the link between MS and the Epstein-Barr Virus, known for causing mononucleosis, has been extensively documented. Research suggests that MS may develop when the immune system attacks myelin in the nervous system, confused by molecular similarities between myelin proteins and those produced by EBV. Groundbreaking Research and Vaccine Development A pivotal study tracking American military personnel revealed a significantly higher rate of EBV antibodies in those diagnosed with MS, reinforcing the virus's role in the disease's development. This discovery has led to the exploration of antiviral therapies aimed at reducing EBV in the immune system. Notably, Moderna and the National Institute of Allergy and Infectious Diseases are at the forefront, developing vaccines focused on the gp350 protein of EBV. Though the journey to a comprehensive solution is ongoing, these efforts represent a significant leap towards potentially eradicating MS. The Road Ahead As the scientific community rallies behind these promising developments, the implications for MS treatment and prevention are profound. However, the effectiveness of these vaccines in preventing MS remains to be fully assessed, with years of research ahead. The potential for a world where MS can be prevented or substantially mitigated is on the horizon, thanks to these innovative strategies targeting the root causes of the disease. The battle against Multiple Sclerosis is entering a hopeful phase, with groundbreaking approaches offering a glimpse into a future where this devastating disease could be significantly controlled or even eliminated. While challenges remain, the dedication and innovation of researchers worldwide signal a potentially transformative era in MS treatment and prevention.

|
Scooped by
Juan Lama
|
Little is known on the landscape of viruses that reside within our cells, nor on the interplay with the host imperative for their persistence. Yet, a lifetime of interactions conceivably have an imprint on our physiology and immune phenotype. In this work, we revealed the genetic make-up and unique composition of the known eukaryotic human DNA virome in nine organs (colon, liver, lung, heart, brain, kidney, skin, blood, hair) of 31 Finnish individuals. By integration of quantitative (qPCR) and qualitative (hybrid-capture sequencing) analysis, we identified the DNAs of 17 species, primarily herpes-, parvo-, papilloma- and anello-viruses (>80% prevalence), typically persisting in low copies (mean 540 copies/ million cells). We assembled in total 70 viral genomes (>90% breadth coverage), distinct in each of the individuals, and identified high sequence homology across the organs. Moreover, we detected variations in virome composition in two individuals with underlying malignant conditions. Our findings reveal unprecedented prevalences of viral DNAs in human organs and provide a fundamental ground for the investigation of disease correlates. Our results from post-mortem tissues call for investigation of the crosstalk between human DNA viruses, the host, and other microbes, as it predictably has a significant impact on our health. Published (April 24, 2023)in Nucleic Acids Research: https://doi.org/10.1093/nar/gkad199

|
Scooped by
Juan Lama
|
Researchers found associations between certain viral illnesses and the risk of Alzheimer’s and other neurodegenerative diseases. Neurodegenerative diseases can damage different parts of the nervous system, including the brain. This may lead to problems with thinking, memory, and/or movement. Examples include Alzheimer’s disease (AD), multiple sclerosis (MS), and Parkinson’s disease (PD). These diseases tend to happen late in life. There are few effective treatments. Previous findings have suggested that viruses may play a role in certain neurodegenerative diseases. For example, a recent study found a link between Epstein-Barr virus infection and the risk of MS. There are also concerns about cognitive impacts from SARS-CoV-2, the virus that causes COVID-19. A research team led by Drs. Mike Nalls, Kristin Levine, and Hampton Leonard of NIH's Center for Alzheimer’s and Related Dementias examined links between viruses and neurodegenerative disease more generally. To do so, they analyzed data from the FinnGen project. This is a repository of biomedical data, or biobank, from more than 300,000 people in Finland. The team searched the biobank for people who had been diagnosed with one of six different conditions: AD, amyotrophic lateral sclerosis (ALS), generalized dementia, vascular dementia, PD, and MS. They then checked how many had been hospitalized for a viral illness before. To confirm their findings, they looked for the same associations in the UK Biobank, which contains data from almost 500,000 people in the United Kingdom. Results appeared in Neuron on January 19, 2023. The researchers found 45 associations between viruses and neurodegenerative diseases in FinnGen. Of these, 22 also appeared in the UK Biobank. The strongest association was between viral encephalitis—brain inflammation caused by a virus—and AD. A person with viral encephalitis in the FinnGen database was 30 times as likely to be diagnosed with AD as someone without encephalitis. Results were similar in the UK Biobank; people with viral encephalitis were 22 times as likely to develop AD as those without. The team also found, in FinnGen, the association between Epstein-Barr virus and MS that was described before. The association wasn’t seen in the UK Biobank, but this may reflect how the different biobanks use hospital diagnostic codes; Epstein-Barr viruses are common and so often not noted. Influenza with pneumonia was associated with all the neurodegenerative diseases except MS. The researchers only included cases of influenza severe enough to need hospitalization in the study. Thus, these associations only apply to the most severe cases of influenza. FinnGen contains data on the same people over time. The team used this to examine how the associations depended on the time since infection. They found that some viral infections were associated with increased risk of neurodegenerative disease as much as 15 years later. The researchers note that vaccines exist for some of the viruses they identified. These include influenza, varicella-zoster (which causes chickenpox and shingles), and certain pneumonia-causing viruses. Vaccination might thus reduce some of the risk of the conditions they examined. “The results of this study provide researchers with several new critical pieces of the neurodegenerative disorder puzzle,” Nalls says. “In the future, we plan to use the latest data science tools to not only find more pieces but also help researchers understand how those pieces, including genes and other risk factors, fit together.”
|

|
Scooped by
Juan Lama
|
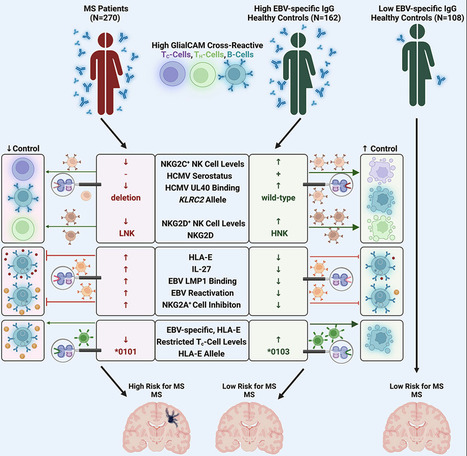
Highlights - Control of autoimmunity by NKG2C+ NK cell responses is severely impaired in MS patients
- MS-patient-derived GlialCAM-specific cells evade control via inhibitory HLA-E/NKG2A axis
- MS patients are predominantly infected with EBV variants that highly upregulate HLA-E
- Specific cytotoxic T cell responses can control EBV-infected GlialCAM-specific B cells
Summary Multiple sclerosis (MS) is a demyelinating disease of the CNS. Epstein-Barr virus (EBV) contributes to the MS pathogenesis because high levels of EBV EBNA386–405-specific antibodies cross react with the CNS-derived GlialCAM370–389. However, it is unclear why only some individuals with such high autoreactive antibody titers develop MS. Here, we show that autoreactive cells are eliminated by distinct immune responses, which are determined by genetic variations of the host, as well as of the infecting EBV and human cytomegalovirus (HCMV). We demonstrate that potent cytotoxic NKG2C+ and NKG2D+ natural killer (NK) cells and distinct EBV-specific T cell responses kill autoreactive GlialCAM370–389-specific cells. Furthermore, immune evasion of these autoreactive cells was induced by EBV-variant-specific upregulation of the immunomodulatory HLA-E. These defined virus and host genetic pre-dispositions are associated with an up to 260-fold increased risk of MS. Our findings thus allow the early identification of patients at risk for MS and suggest additional therapeutic options against MS. Published in Cell (December 12, 2023):

|
Scooped by
Juan Lama
|
The Epstein-Barr virus (EBV) is easily spread through bodily fluids, primarily saliva, such as kissing, shared drinks or using the same eating utensils. Not surprisingly then, EBV is also among the most ubiquitous of viruses: More than 90% of the world’s population has been infected, usually during childhood. EBV causes infectious mononucleosis and similar ailments, though often there are no symptoms. Most infections are mild and pass, but the virus persists in the body, becoming latent or inactive, sometimes reactivating. Long-term latent infections are associated with several chronic inflammatory conditions and multiple cancers. In a new paper, published April 12, 2023 in the journal Nature, researchers at University of California San Diego, UC San Diego Moores Cancer Center and Ludwig Cancer Research at UC San Diego, describe for the first time how the virus exploits genomic weaknesses to cause cancer while reducing the body’s ability to suppress it. These findings show “how a virus can induce cleavage of human chromosome 11, initiating a cascade of genomic instability that can potentially activate a leukemia-causing oncogene and inactivate a major tumor suppressor,” said senior study author Don Cleveland, PhD, Distinguished Professor of Medicine, Neurosciences and Cellular and Molecular Medicine at UC San Diego School of Medicine. “It’s the first demonstration of how cleavage of a ‘fragile DNA’ site can be selectively induced.” Throughout every person’s genome or full set of genes are fragile sites, specific chromosomal regions more likely to produce mutations, breaks or gaps when replicating. Some are rare, some are common; all are associated with disorders and disease, sometimes heritable conditions, sometimes not, such as many cancers. In the new study, Cleveland and colleagues focus on EBNA1, a viral protein that persists in cells infected with EBV. EBNA1 was previously known to bind at a specific genomic sequence in the EBV genome at the origin of replication. The researchers found that EBNA1 also binds a cluster of EBV-like sequences at a fragile site on human chromosome 11 where increasing abundance of the protein triggers chromosomal breakage. Other prior research has shown that EBNA1 inhibits p53, a gene that plays a key role in controlling cell division and cell death. It also suppresses tumor formation when normal. Mutations of p53, on the other hand, are linked to cancer cell growth. When the scientists examined whole-genome sequencing data for 2,439 cancers across 38 tumor types from the Pan-Cancer Analysis of Whole Genomes project, they found that cancer tumors with detectable EBV revealed higher levels of chromosome 11 abnormalities, including 100% of the head and neck cancer cases. “For a ubiquitous virus that is harmless for the majority of the human population, identifying at-risk individuals susceptible to the development of latent infection-associated diseases is still an ongoing effort,” said the study’s first author Julia Li, PhD, a postdoctoral fellow in Cleveland’s lab. “This discovery suggests that susceptibility to EBNA1-induced fragmentation of chromosome 11 depends on the control of EBNA1 levels produced in latent infection, as well as the genetic variability in the number of EBV-like sequences present on chromosome 11 in each individual. Going forward, this knowledge paves the way for screening risk factors for the development of EBV-associated diseases. Moreover, blocking EBNA1 from binding at this cluster of sequences on chromosome 11 can be exploited to prevent the development of EBV-associated diseases.” Published in Nature (April 12, 2023):
|



 Your new post is loading...
Your new post is loading...