Dyno Therapeutics announces a publication in Science demonstrating the power of a comprehensive machine-guided approach to improve adeno-associated virus (AAV) capsids for gene therapies. Working to surpass the few naturally occurring AAV capsids currently in use, the authors synthesized large libraries of capsids and discovered changes that improve key properties preventing current AAVs from optimal therapeutic function. Dyno is a biotechnology company pioneering the use of artificial intelligence in gene therapy.
AAV capsids are presently the most commonly used vector for gene therapy because of their established ability to deliver genetic material to patient organs with a proven safety profile. However, there are only a few naturally occurring AAV capsids, and they are deficient in essential properties for optimal gene therapy, such as targeted delivery, evasion of the immune system, higher levels of viral production, and greater transduction efficiency. Starting at Harvard in 2015, the authors set out to overcome the limitations of current capsids by developing new machine-guided technologies to rapidly and systematically engineer a suite of new, improved capsids for widespread therapeutic use.
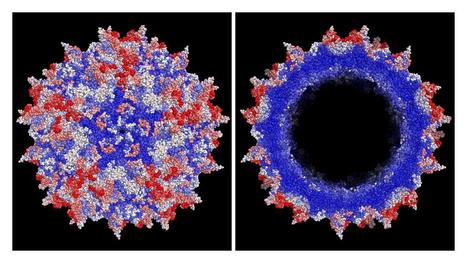
In the research published in Science, the authors demonstrate the advance of their unique machine-guided approach to AAV engineering. Previous approaches have been limited by the difficulty of altering a complex capsid protein without breaking its function and by the general lack of knowledge regarding how AAV capsids interact with the body. Historically, rather than addressing this challenge directly, the most popular approaches to capsid engineering have taken a roundabout solution: generating libraries of new capsids by making random changes to the protein. However, since most random changes to the capsid actually result in decreased function, such random libraries contain few viable capsids, much less improved ones. Recognizing the limitation of conventionally generated capsid libraries, the authors implemented a machine-guided approach that gathered a vast amount of data using new high-throughput measurement technologies to teach them how to build better libraries and, ultimately, lead to synthetic capsids with optimized delivery properties. Focusing on the AAV2 capsid, the authors generated a complete landscape of all single codon substitutions, insertions and deletions, then measured the functional properties important for in vivo delivery. They then used a machine-guided approach, leveraging these data to efficiently generate diverse libraries of AAV capsids with multiple changes that targeted the mouse liver and that outperformed AAVs generated by conventional random mutagenesis approaches. In the process, the authors' systematic efforts unexpectedly revealed the existence of a previously-unrecognized protein encoded within the sequence of all the most popular AAV capsids, which they termed membrane-associated accessory protein (MAAP). The authors believe that the protein plays a role in the natural life cycle of AAV.
"This is just the beginning of machine-guided engineering of AAV capsids to transform gene therapy," underscores co-author Sam Sinai, Ph.D., Lead Machine Learning Scientist and co-founder of Dyno Therapeutics. "The success of the simple linear models used in this study has led us to pursue more data and higher capacity machine learning models, where the potential for improvement in capsid designs feels boundless."
"The results in the Science publication demonstrate, for the first time, the power of linking a comprehensive set of advanced techniques - large scale DNA synthesis, pooled in vitro and in vivo screens, next-generation sequencing readouts, and iterative machine-guided capsid design - to generate optimized synthetic AAV capsids," explains co-first and co-corresponding author Eric D. Kelsic, Ph.D., CEO and co-founder of Dyno Therapeutics. "At Dyno, our team is committed to advancing these technologies to identify capsids that meet the urgent needs of patients who can benefit from gene therapies."....
Published in Science (29 November, 2019):



 Your new post is loading...
Your new post is loading...