Your new post is loading...

|
Scooped by
Juan Lama
|
- The US FDA has revoked the emergency use authorization for Johnson & Johnson Janssen unit's COVID vaccine. Read more here.
- Janssen voluntarily requested the withdrawal on May 22.
- The company noted that the last lots purchased by the US Government have expired, there is no additional demand in the US, and they do not intend to update the strain composition for emerging variants.
- mRNA vaccines made by Moderna (MRNA) and Pfizer (PFE)/BioNTech (BNTX) account for almost all of the COVID shots administered in the US now.
FDA Letter June 1, 2023: https://www.fda.gov/media/169003/download

|
Scooped by
Juan Lama
|
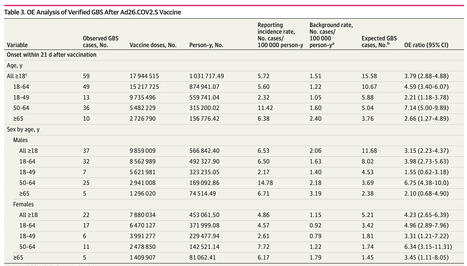
Question Are Ad26.COV2.S (Janssen), BNT162b2 (Pfizer-BioNTech), or mRNA-1273 (Moderna) COVID-19 vaccines associated with Guillain-Barré syndrome (GBS) within 21 or 42 days after vaccination? Findings This cohort study of 487 651 785 COVID-19 vaccine doses found that in observed-to-expected analyses, the observed number of GBS reports was higher than expected based on background rates within 21 and 42 days after vaccination for Ad26.COV2.S but not BNT162b2 or mRNA-1273. GBS reporting rates within 21 and 42 days of Ad26.COV2.S vaccination were 9 to 12 times higher than after BNT162b2 or mRNA-1273 vaccination. Meaning These findings suggest that Ad26.COV2.S vaccination was associated with GBS and that GBS after BNT162b2 and mRNA-1273 may represent background incidence. Importance Because of historical associations between vaccines and Guillain-Barré syndrome (GBS), the condition was a prespecified adverse event of special interest for COVID-19 vaccine monitoring. Objective To evaluate GBS reports to the Vaccine Adverse Event Reporting System (VAERS) and compare reporting patterns within 21 and 42 days after vaccination with Ad26.COV2.S (Janssen), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) COVID-19 vaccines. Design, Setting, and Participants This retrospective cohort study was conducted using US VAERS reports submitted during December 2020 to January 2022. GBS case reports verified as meeting the Brighton Collaboration case definition for GBS in US adults after COVID-19 vaccination were included. Exposures Receipt of the Ad26.COV2.S, BNT162b2, or mRNA-1273 COVID-19 vaccine. Main Outcomes and Measures Descriptive analyses of GBS case were conducted. GBS reporting rates within 21 and 42 days after Ad26.COV2.S, BNT162b2, or mRNA-1273 vaccination based on doses administered were calculated. Reporting rate ratios (RRRs) after receipt of Ad26.COV2.S vs BNT162b2 or mRNA-1273 within 21- and 42-day postvaccination intervals were calculated. Observed-to-expected (OE) ratios were estimated using published GBS background rates. Results Among 487 651 785 COVID-19 vaccine doses, 17 944 515 doses (3.7%) were Ad26.COV2.S, 266 859 784 doses (54.7%) were BNT162b2, and 202 847 486 doses (41.6%) were mRNA-1273. Of 295 verified reports of individuals with GBS identified after COVID-19 vaccination (12 Asian [4.1%], 18 Black [6.1%], and 193 White [65.4%]; 17 Hispanic [5.8%]; 169 males [57.3%]; median [IQR] age, 59.0 [46.0-68.0] years), 275 reports (93.2%) documented hospitalization. There were 209 and 253 reports of GBS that occurred within 21 days and 42 days of vaccination, respectively. Within 21 days of vaccination, GBS reporting rates per 1 000 000 doses were 3.29 for Ad26.COV.2, 0.29 for BNT162b2, and 0.35 for mRNA-1273 administered; within 42 days of vaccination, they were 4.07 for Ad26.COV.2, 0.34 for BNT162b2, and 0.44 for mRNA-1273. GBS was more frequently reported within 21 days after Ad26.COV2.S than after BNT162b2 (RRR = 11.40; 95% CI, 8.11-15.99) or mRNA-1273 (RRR = 9.26; 95% CI, 6.57-13.07) vaccination; similar findings were observed within 42 days after vaccination (BNT162b2: RRR = 12.06; 95% CI, 8.86-16.43; mRNA-1273: RRR = 9.27; 95% CI, 6.80-12.63). OE ratios were 3.79 (95% CI, 2.88-4.88) for 21-day and 2.34 (95% CI, 1.83-2.94) for 42-day intervals after Ad26.COV2.S vaccination and less than 1 (not significant) after BNT162b2 and mRNA-1273 vaccination within both postvaccination periods. Conclusions and Relevance This study found disproportionate reporting and imbalances after Ad26.COV2.S vaccination, suggesting that Ad26.COV2.S vaccination was associated with increased risk for GBS. No associations between mRNA COVID-19 vaccines and risk of GBS were observed.

|
Scooped by
Juan Lama
|
Once dismissed as less effective, the vaccine now seems to be preventing infections and illness about as well as the two mRNA options. Roughly 17 million Americans received the Johnson & Johnson Covid vaccine, only to be told later that it was the least protective of the options available in the United States. But new data suggest that the vaccine is now preventing infections, hospitalizations and deaths at least as well as the Pfizer-BioNTech and Moderna vaccines. The reasons aren’t clear, and not all experts are convinced that the vaccine has vindicated itself. But the accumulating data nonetheless offer considerable reassurance to recipients of the vaccine and, if confirmed, have broad implications for its deployment in parts of the world. In Africa, for example, distribution of a single-dose vaccine that can be refrigerated for months is by far the most practical option. Johnson & Johnson has at least temporarily shut down the only plant making usable batches of the vaccine. But the South Africa-based Aspen Pharmacare is gearing up to supply large quantities to the rest of the continent. Only about 13 percent of Africans are fully vaccinated, and only about 1 percent have received a booster dose. “In the setting of Africa, where we have the need to quickly get vaccines out, the single dose is very exciting,” said Linda Gail-Bekker, director of the Desmond Tutu H.I.V. Center at the University of Cape Town, who has studied the vaccine’s effectiveness in South Africa. The Johnson & Johnson vaccine was billed as an attractive option for communities with limited access to health care, including some within the United States, because of its ease of delivery and mild side effects. But it has had a bumpy journey. The shot seemed to produce a weaker initial immune response, and more people who got the single-dose vaccine had breakthrough infections, compared with those who got two doses of Pfizer or Moderna, the mRNA vaccines. In April, federal health officials in the United States and in South Africa paused the J.&J. vaccine’s distribution as they examined reports of a rare blood-clotting disorder in women. Though both countries resumed the rollouts soon after, the vaccine’s reputation never fully recovered. But the notion that the vaccine is inferior has grown outdated, some experts said: More recent data suggest that it has more than held its own against its competitors. “We’ve been aware that J.&J. has been kind of downgraded in people’s minds,” Dr. Gail-Bekker said. But “it punches above its weight for a single-dose vaccine.” Until last June, the cumulative data from the C.D.C. showed that immunization with the Moderna vaccine resulted in the lowest rates of breakthrough infections; those who got Johnson & Johnson saw the highest rates, with Pfizer-BioNTech somewhere in the middle. During the summer months, the gaps — particularly between J.&J. and Pfizer — began to narrow. By now, all the vaccines seem to be performing about equally well against coronavirus infections; in fact, Johnson & Johnson appears to be holding up slightly better. As of Jan. 22, the latest data available, unvaccinated people were 3.2 times as likely to become infected as those who received the single-dose Johnson & Johnson vaccine; they were 2.8 times as likely to become infected as those who received two doses of the Moderna vaccine and 2.4 times as likely as those with two doses of Pfizer-BioNTech. Overall, then, the Johnson & Johnson vaccine appeared to be somewhat more protective against infection than the two alternatives. Among Americans who got booster doses, all the vaccines appeared to have roughly the same effectiveness against infection. A booster shot did not add much to Johnson & Johnson’s previous level of protection (although the data do not indicate who received which type of booster shot). The data were collected by the C.D.C. from 29 jurisdictions, representing 67 percent of the population....

|
Scooped by
Juan Lama
|
Two reports released Thursday show that people who get booster doses of Johnson & Johnson's Janssen vaccine are well protected against severe disease and hospitalization from the Omicron variant of coronavirus, the company said. Researchers said the findings indicate that most of the Covid-19 vaccines will protect people against the worst outcomes from infection -- and show some of the emphasis on how the various vaccines affect immune system components called antibodies may be misleading. One real-life study from South Africa showed vaccine effectiveness against hospitalization from Covid-19 rose to 85% after a booster dose of the J&J vaccine, even after the Omicron variant was circulating. And a lab-based study in the US indicated the vaccine stimulates a strong immune response from cells known as T-cells, which protect people against severe disease even if they don't block the virus entirely from infecting the body. Results from both studies were released by the company in a statement but the South African team posted findings online as a preprint and the results are being submitted to a peer-reviewed journal, the researchers said. Linda-Gail Bekker of the University of Cape Town and other researchers including a team at the South African Medical Research Council helped examine the results of an ongoing study of the J&J vaccine there. They looked at results from 69,000 health care workers. "We observed that vaccine effectiveness for hospitalization increased over time since booster dose, from 63% to 84% and then 85%," they wrote. The booster was given between six and nine months after the first dose of the vaccine, also known as Ad26.COV.2. "This data is important given the increased reliance on the Ad26.COV.2 vaccine in Africa," they wrote. "Even before you factor in the increased infectiousness of Omicron, we have to remember that healthcare workers on the frontlines are at a greatly increased risk of being affected by COVID-19 in the first place," Dr. Glenda Gray, president and CEO of the SAMRC, said in a statement. "We are therefore encouraged to see that boosting with the Johnson & Johnson COVID-19 vaccine regimen provides strong protection in a challenging real-world setting where there is an elevated risk of exposure -- not just to COVID-19, but to the highly transmissible Omicron variant." Separately, Dr. Dan Barouch and colleagues at the Beth Israel Deaconess Medical Center in Boston looked at blood taken from 65 vaccinated volunteers and tested it against the Omicron variant. They looked at both antibodies -- the first line of defense against infection -- and T-cells. Using the J&J vaccine as a booster for people who originally got two doses of Pfizer/BioNTech's vaccine generated a 41-fold increase in neutralizing antibodies and a five-fold increase in the CD8 killer T cells that destroy cells infected by the virus. That stops the virus from replicating and spreading. Boosting with the Pfizer vaccine generated a 17-fold increase in neutralizing antibodies and a 1.4-fold increase in CD8 T cells four weeks later, they found. "These data are important and these data are hopeful," Barouch told CNN. They indicate that all Covid-19 vaccines can protect people from severe disease and death, even from the Omicron variant with all its mutations, he said. "It has substantial global significance that goes well beyond J&J and goes well beyond South Africa," he added. South African researchers reported in the New England Journal of Medicine on Wednesday that protection against hospitalization from two doses of Pfizer's vaccine fell to about 70% when Omicron was circulating compared to 93% a few weeks earlier, when Delta was dominant in South Africa. Barouch said many studies look only at antibodies, which can stop the virus from infecting cells at all. He said the T-cell response, which is trickier to measure, is important in providing long-term protection from severe disease. "There's confusion -- not just in the media and the public but also among doctors and scientists -- that only neutralizing antibodies equate with protection, and that's just not true," he said. "What we are seeing with 70% protection with Pfizer and now 85% protection with J&J -- which is occurring at very low levels of neutralizing antibodies -- strongly suggests T-cell responses are important in the protection that we are seeing." When viruses infect cells, they take over their internal machinery and turn them into little virus factories. While antibodies attach to the outside of viruses and stop them from ever docking to cells, T-cells seek out and destroy cells after they are infected. This may not completely stop infection, but it elps stop virus from spreading and causing severe illness. Dr. Mathai Mammen, global head of research and development at J&J's Janssen vaccine arm, agreed. "We believe that the protection could be due to the robust T-cell responses induced by the Johnson & Johnson COVID-19 vaccine. Furthermore, these data suggest that Omicron is not affecting the T-cell responses generated by our vaccine," he said in a statement. The findings may also reassure the millions of people who got the Janssen vaccine. Earlier this month, the US Centers for Disease Control and Prevention endorsed the Moderna and Pfizer/BioNTech vaccines over J&J's, saying the two mRNA vaccines worked better and more safely than J&J's, which is linked with a rare type of blood clotting event. The mRNA vaccines use a newer technology that involves genetic material called messenger RNA, carried into the body by simple fatty compounds called lipids. J&J's vaccine is an adenoviral vector vaccine, which uses a crippled common cold virus to carry the genetic instructions into the body. J&J said the vaccine's design is deliberately meant to elicit a robust T-cell response. Johnshon & Johnson Press Release (Dec. 30, 20210: https://www.jnj.com/johnson-johnson-covid-19-vaccine-demonstrates-85-percent-effectiveness-against-hospitalization-in-south-africa-when-omicron-was-dominant

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration cleared a path for millions more Americans to receive Covid-19 vaccine booster shots, as the nation looks to bolster its defenses and prevent another virus surge. The agency said in a statement on Wednesday that Moderna Inc. vaccine recipients 65 and over can receive a third shot, as can adults 18 and up who are at high risk of severe Covid or with frequent institutional or occupational exposure to the virus that causes the disease. Additionally, all J&J recipients 18 and older are eligible for a booster shot at least two months after receiving their first dose. The agency also allowed each of the available Covid vaccines to be used as a booster dose for eligible individuals following completion of a primary vaccination with a different vaccine. The moves will mean the U.S. has a bigger toolkit to try to limit a potential winter virus rebound. The summer’s delta-variant fueled spike in infections helped increase urgency to make boosters available, and health officials across the U.S. are eager to forestall a rebound in cases that could cripple hospitals and disrupt work and school this winter. FDA officials indicated they would also move quickly to expand eligibility for booster shots as more data become available or if breakthrough cases start to rise in younger adults. “We will not hesitate to drop this age range as we see that that benefit clearly outweighs the risk,” said Peter Marks, the head of the agency’s Center for Biologics Evaluation and Research, during a media briefing following the announcement. The clearances came after a panel of expert advisers to the FDA unanimously backed the Moderna and J&J booster regimens in two days of meetings last week. Regulators have now signed off on boosters for all three coronavirus vaccines available in the U.S. Last month, the FDA said people 65 and over and others who are at heightened risk of severe Covid were eligible for a booster dose of the vaccine developed by Pfizer Inc. and BioNTech SE. Moderna shares climbed 0.8% in after-hours trading in New York, while J&J shares gained 0.4% and Pfizer shares rose 0.2%. U.S.-traded shares of Germany-based BioNTech gained 0.9%. Smaller Dose The Moderna booster shot authorized by the FDA is half the dose that is given in the initial two-shot series, and it should be given at least six months after the initial inoculation, regulators said. The FDA said that a single booster dose of the Pfizer vaccine may be given at least 6 months after completing the primary series to people 18 to 64 with frequent institutional or occupational exposure to the coronavirus. In permitting mixing and matching, the FDA is allowing J&J vaccine recipients to receive an additional dose of any cleared vaccine after two months. Likewise, recipients of Moderna and Pfizer who are eligible for a booster would receive their booster, including J&J’s shot, at least six months after their initial immunization regimen. Marks said during the call with reporters that different combinations produce different antibody levels in the short term, but it isn’t clear what that means in terms of actual long-term protection. Seen as a convenient, effective alternative to two-shot messenger RNA vaccines, J&J’s single-shot immunization has seen far less use in the U.S., in part because it isn’t as effective. The drugmaker has also experienced manufacturing problems that limited the shot’s distribution. The decision to allow mixing will create greater flexibility and is beneficial to global public health, Paul Stoffels, J&J’s chief scientific officer, said in a statement. Before the Moderna and J&J booster shots can be administered, the Centers for Disease Control and Prevention’s Advisory Panel on Immunization Practices will make further recommendations about who should receive them. The panel is scheduled to discuss boosters on Thursday. The next big milestone for the U.S. immunization effort looms next week, when the FDA advisory panel is expected to weigh Pfizer’s proposed Covid vaccine for children ages 5 to 11. If authorized, it could begin to roll out to pediatricians’ offices and drugstores as soon as next month. — With assistance by Jeannie Baumann, and Riley Griffin FDA Press Release (Oct. 20, 2021) available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines

|
Scooped by
Juan Lama
|
Vaccine advisers to the US Food and Drug Administration voted unanimously Friday to recommend a booster dose of Johnson & Johnson's vaccine at least two months after people get the first dose. The FDA's Vaccines and Related Biological Products Advisory Committee voted 19-0 to recommend the extra dose for all recipients of the J&J Janssen vaccine, 18 and older. They asked to simplify the original question being posed by the FDA, which had asked the committee to say whether the data showed that waiting six months or longer after getting the first shot would provide an even stronger immune response. The FDA will now consider the committee's advice. Then the US Centers for Disease Control and Prevention's vaccine advisers will be asked to consider it. Johnson & Johnson says studies have shown boosting at two or six months can bring that effectiveness up to 94% and it says its effectiveness does not wane over time in the same way that effectiveness from Pfizer's vaccine does. But the Johnson & Johnson vaccine has not been shown to be as protective as either the Pfizer or Moderna vaccines, noted VRBPAC chair Dr. Arnold Monto, a professor of public health and epidemiology at the University of Michigan. "So there is some urgency here to do something," he told the meeting. And the CDC's Dr. Amanda Cohn told the meeting that various studies suggested real-world efficacy of J&J's vaccine was anywhere between 50% and 68%. "Regardless of whether or not there been waning or this is the true effectiveness after a single dose, the effectiveness or protection of a single dose of the J&J vaccine is not equivalent to protection at this time with either two doses of an mRNA vaccine and certainly not in those groups who have now been authorized to receive a booster dose of an mRNA vaccine," Cohn said. Members of the committee said while there was not much data to show whether the efficacy of the Janssen vaccine was waning -- or was strong to begin with -- they agreed people should be given the opportunity for a booster. "I would say I agree a second dose booster is needed to boost immunity back to the 90-plus range," Dr. Archana Chatterjee, a pediatric infectious diseases expert at Rosalind Franklin University in Chicago, said before the vote. The FDA has already given EUA to a booster for Pfizer's vaccine for people who are six months out from their first two shots who are also either 65 or older or who are at least 18 and have a higher risk of severe disease because of pre-existing conditions or because of work or living conditions. And Americans are already flocking to get those boosters. Data from the US Centers for Disease Control and Prevention show close to 5% of fully vaccinated people -- about 9 million people -- have received booster shots. On Thursday, VRBPAC members voted unanimously to recommend booster doses of Moderna's vaccine to the same groups. If the FDA gives emergency use authorization to Moderna or Johnson & Johnson boosters, CDC vaccine advisers will meet to discuss which groups to recommend them to. Typically, shots can be administered once the CDC director signs off on the recommendation. CDC's Advisory Committee on Immunization Practices is scheduled to discuss boosters on October 21.

|
Scooped by
Juan Lama
|
After years in development, the floodgates for RSV vaccines may open up soon, with more and more Big Pharmas piling on data. Now, Johnson & Johnson’s Janssen is adding to the fray, announcing efficacy as high as 80% in preventing severe infections in a mid-stage clinical trial. Janssen revealed the proof-of-concept data at IDWeek Saturday, showcasing a trial called CYPRESS that featured more than 5,700 people aged 65 years and up randomized to receive the RSV vaccine candidate or placebo. The main goal was the preventing the occurrence of lower respiratory tract disease as a result of RSV infection. A dozen secondary endpoints looked for adverse events, neutralizing antibodies and other outcomes. Protection from Janssen’s vaccine ranged from 80% in preventing severe lower respiratory tract infection caused to 70% for more mild cases. The study concluded that the vaccine was effective in protecting against RSV-caused infections through the first viral season. While the CYPRESS data presented at IDWeek are from one RSV season, Macaya Douoguih, Ph.D., head of clinical development and medical affairs in Janssen, said trial participants will be tracked for a second season to see how long the efficacy lasts. “You never know when you need to boost from the first season so that will hopefully help give us an indication of what's needed there,” Douoguih said. Before presenting the data at the conference, Janssen had already announced Sept. 29 that the RSV vaccine would be moved to a phase 3 trial called EVERGREEN on the strength of the phase 2 data. This late-stage trial will have 23,000 participants with an expanded age group of 60 and up. Trial volunteers will be tracked for at least two seasons. Douoguih added that CYPRESS showed a 13.5-fold increase in antibody titers that occurred 14 days after vaccination, which reflects an immune response. The vaccine was also well tolerated by all participants with no safety signals. “We think that these results are quite solid,” said Douoguih. There is no vaccine available for RSV, which according to the Centers for Disease Control and Prevention causes an average of 58,000 hospitalizations a year in the U.S., with 100-500 deaths among children younger than five years old as well as 177,000 hospitalizations with 14,000 deaths among adults aged 65 years or older. Janssen is also testing the RSV candidate in children. A phase 1 has been completed while two midstage studies are underway. Janssen’s shot could one day go up against Pfizer's candidate, which showed 100% efficacy against mild to moderate cases though in people under 50 and in a challenge trial, where study volunteers are purposely infected with the virus and then monitored. The New York-based pharma is yet to provide details on severe cases from that 62-person trial. GlaxoSmithKline and Moderna are also advancing candidates. GSK has seen a mixed journey in clinic trials, having abandoned its candidate in children back in July. The U.K. pharma is moving ahead with a maternal study to see whether vaccination can protect the mother and child. Pfizer too is investigating its candidate in pregnant women. Janssen's Press Release (Sept. 29, 2021): https://www.jnj.com/janssen-announces-start-of-phase-3-trial-for-investigational-respiratory-syncytial-virus-rsv-vaccine-in-older-adults

|
Scooped by
Juan Lama
|
Vaccine effectiveness against COVID-19 hospitalization among U.S. adults without immunocompromising conditions was highest for the Moderna vaccine compared with the other two available COVID-19 vaccines, researchers reported in MMWR. “Three COVID-19 vaccines are authorized or approved for use among adults in the United States,” Wesley H. Self, MD, associate professor at the Vanderbilt University School of Medicine, and colleagues wrote. “Current guidelines from FDA and CDC recommend vaccination of eligible persons with one of these three products, without preference for a specific vaccine.” To better assess vaccine effectiveness (VE) of the three available COVID-19 vaccines in the U.S. in preventing COVID-19 hospitalization, Self and colleagues conducted a case-control analysis among 3,689 adults aged 18 years and older who were hospitalized at 21 U.S. hospitals between March 11 and Aug. 15, 2021. According to the study, an additional analysis compared serum antibody levels to SARS-CoV-2 among 100 healthy volunteers enrolled at three hospitals 2 to 6 weeks after full vaccination with the Moderna, Pfizer-BioNTech or Johnson & Johnson vaccines. Overall, the study demonstrated that VE against COVID-19 hospitalizations was higher for the Moderna vaccine (93%; 95% CI, 91%-95%) than for the Pfizer-BioNTech vaccine (88%; 95% CI, 85%-91%), whereas VE for both of the messenger RNA vaccines was higher than that of the Johnson & Johnson vaccine (71%; 95% CI, 56%-81%). Additionally, the study showed that protection from the Pfizer-BioNTech vaccine declined 4 months after vaccination. Researchers added that post-vaccination anti-spike IgG and anti-receptor binding domain IgG levels were significantly lower in participants who were vaccinated with the Johnson & Johnson vaccine compared with the Moderna or Pfizer-BioNTech vaccines. Research cited published in MMWR (Sept.17, 2021): http://dx.doi.org/10.15585/mmwr.mm7038e1

|
Scooped by
Juan Lama
|
J&J's HIV vaccine, using the same technology as its COVID vaccine, failed to meet its goal of reducing the chance of HIV infection by 50%. An HIV vaccine using the same basic technology as Johnson & Johnson’s Covid shot failed to prevent infection, the company said Tuesday, dealing yet another blow to efforts to create a vaccine against the virus. The study, called Imbokodo, enrolled 2,600 women in southern Africa who were at very high risk of HIV infection. J&J and its partners, including the National Institutes of Health and the Bill & Melinda Gates Foundation, launched the study in 2017 and announced that all participants had received either a vaccine or a placebo last year. The goal of the vaccine was not to completely prevent infection, but to reduce the chance of infection by half. “If a vaccine is 50% efficacious it can curb the future of the HIV pandemic,” said Paul Stoffels, J&J’s chief scientific officer and, before that, an HIV researcher. He said that the actual efficacy seen was 25.2%, meaning those that received the vaccine had their odds of becoming infected reduced that much compared to the placebo group 24 months after the first dose. That difference was not statistically significant, indicating that it is possible the result is due to chance. A second study, called Mosaico, that is testing a somewhat different vaccine regimen in men who have sex with men and transgender people in the Americas and Europe, will continue. “The development of a safe and effective vaccine to prevent HIV infection has proven to be a formidable scientific challenge,” Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, said in a statement. “Although this is certainly not the study outcome for which we had hoped, we must apply the knowledge learned from the Imbokodo trial and continue our efforts to find a vaccine that will be protective against HIV.” Scientists have been trying for decades to develop an HIV vaccine. After a Merck vaccine failed to prove effective in 2007, researchers looked back at the data and found it raised the risk of people developing the disease. Hopes were buoyed by a 2009 study in Thailand, which showed limited but significant efficacy, reducing the rate of infection by about 30%. But last year, an effort combining vaccines from Sanofi and GlaxoSmithKline also failed to prove effective. J&J had repeatedly expressed optimism about its vaccine. In 2015, Johan Van Hoof, who leads J&J’s vaccine R&D, pointed to data showing that, in animals, the vaccine could reduce infection by 90%, “suggesting it might be a real breakthrough with regard to a future HIV vaccine.” On calls with financial analysts in 2020 and 2021, Stoffels listed the HIV project among the company’s vaccine efforts, calling it “very encouraging.” When the trial began five years ago, Fauci had said “the development and delivery of a preventive HIV vaccine that is safe and at least moderately effective would help bring about a durable end to the HIV/AIDS pandemic.” Imbokodo means “rock” in isiZulu, and refers to a proverb about women’s strength and the need for community. In the study, 63 of 1,109 volunteers in the placebo group developed HIV, while 51 of the 1,079 volunteers who received the vaccine developed HIV. That difference leaves a great deal of uncertainty as to whether there was an effect. The 95% confidence interval, used by researchers to define a range of likely outcomes, ranged from -10.5% to 49.3%. However, J&J said in its release that no vaccine-related safety issues were identified. Stoffels told STAT that it was clear the vaccine did not increase the risk of HIV. Larry Corey, the principal investigator of the HIV Trials Network, which helped run the study, and a professor at the Fred Hutchinson Cancer Research Center in Seattle, said that he saw the result as a disappointment but also a sign of progress. It had been hoped that non-neutralizing antibodies – those that bound to the virus but did not entirely stop infectivity – would be enough to slow the rate of HIV infection, he said, but it is beginning to appear that vaccine developers will need to figure out how to generate antibodies that neutralize the virus. “It is telling us that non-neutralizing antibodies are not decreasing acquisition and maybe demonstrates how difficult and different HIV is than Covid-19,” Corey said. Like the Covid-19 vaccine that Johnson & Johnson developed, this HIV vaccine delivers genetic code for proteins to a recipient’s cells using a type of virus called an adenovirus, which then makes proteins that the immune system learns to recognize and attack. The strain of adenovirus used, called Ad26, is also used in Johnson & Johnson’s experimental vaccine against respiratory syncytial virus, which can be very serious in infants. The HIV vaccine regimen tested repeated dosing. It was given four times, and included genetic code for a “mosaic” of proteins from different strains of the HIV virus. Patients also received soluble protein injections at the third and fourth visit. The ongoing Mosaico study – the one in the Americas and Europe – uses a different mixture of soluble proteins at the vaccination visits three and four. Stoffels said that this is one reason that the vaccine might perform better in that study. Another is that the volunteers in the Mosaico study are at lower risk of infection, which may make the vaccine’s work less difficult. Corey also said there was hope Mosaico would succeed where Imbokodo failed. He said that the new formulation had led, in earlier studies, to higher levels of antibodies against HIV. Stoffels said that he doesn’t believe that the result should color people’s feelings about J&J’s adenovirus vaccine platform, which, he pointed out, has proven effective against Covid-19 and Ebola. (In Covid-19, the broad use of the vaccine also was linked to a rare but severe side effect that involves both the formation of clots and excessive bleeding. That side effect is so rare even large clinical trials might not detect it.) “It shows again that the HIV virus is a very special virus, very unique, escaping the immune system and finding its way to infect people and it’s very difficult to mount immunity against acquisition of HIV,” Stoffels said. But researchers will continue to try. Moderna recently began human trials of an HIV vaccine that relies on the mRNA technology behind its Covid vaccines. Corey pointed out that even under Covid lockdowns, women in the study still had a 4% chance of contracting HIV. That underscores the need for a vaccine, he said. “Vaccines really do make a difference when you have an effective vaccine, look at what happened with Covid,” Corey said. “I think we can’t give up.”

|
Scooped by
Juan Lama
|
During the last months many countries have started the immunization of millions of people by using vector-based vaccines. Unfortunately, severe side effects became overt during these vaccination campaigns: cerebral venous sinus thromboses (CVST), absolutely rare under normal life conditions, were found as a severe side effect that occured 4-14 days after first vaccinations. Besides CVST, Splanchnic Vein Thrombosis (SVT) was also observed. This type of adverse event has not been observed in the clinical studies of AstraZeneca, and therefore led immediately to a halt in vaccinations in several european countries. These events were mostly associated with thrombocytopenia, and thus, similar to the well-known Heparin-induced thrombocytopenia (HIT). Meanwhile, scientists have proposed a mechanism to explain this vaccine-induced thrombocytopenia. However, they do not provide a satisfactory explanation for the late thromboembolic events. Here, we present data that may explain these severe side effects which have been attributed to adenoviral vaccines. According to our results, transcription of wildtype and codon-optimized Spike open reading frames enables alternative splice events that lead to C-terminal truncated, soluble Spike protein variants. These soluble Spike variants may initiate severe side effects when binding to ACE2-expressing endothelial cells in blood vessels. In analogy to the thromboembolic events caused by Spike protein encoded by the SARS-CoV-2 virus, we termed the underlying disease mechanism the “Vaccine-Induced Covid-19 Mimicry” syndrome (VIC19M syndrome). Preprint available in Research Square (May 26, 2021): https://assets.researchsquare.com/files/rs-558954/v1/8c30a186-e9e2-47c1-a76c-dc3bdf10c22a.pdf

|
Scooped by
Juan Lama
|
The increasing prevalence of SARS-CoV-2 variants has raised concerns regarding possible decreases in vaccine efficacy. Here, neutralizing antibody titers elicited by mRNA-based and an adenoviral vector-based vaccine against variant pseudotyped viruses were compared. BNT162b2 and mRNA-1273-elicited antibodies showed modest neutralization resistance against Beta, Delta, Delta plus and Lambda variants whereas Ad26.COV2.S-elicited antibodies from a significant fraction of vaccinated individuals were of low neutralizing titer (IC50 <50). The data underscore the importance of surveillance for breakthrough infections that result in severe COVID-19 and suggest the benefit of a second immunization following Ad26.COV2.S to increase protection against the variants. Preprint Available at bioRxiv (July 19, 2021): https://doi.org/10.1101/2021.07.19.452771

|
Scooped by
Juan Lama
|
Federal regulators concluded that the risk of developing the syndrome was low, and that the benefits of the vaccine still strongly outweigh it. The Food and Drug Administration is planning to warn that Johnson & Johnson’s coronavirus vaccine can lead to an increased risk of a rare neurological condition known as Guillain–Barré syndrome, another setback for a vaccine that has largely been sidelined in the United States. Although regulators have found that the chances of developing the condition are low, they appear to be three to five times higher among recipients of the Johnson & Johnson vaccine than among the general population in the United States, according to people familiar with the decision. Federal officials have identified roughly 100 suspected cases of Guillain-Barré disease among recipients of the Johnson & Johnson shot through a federal monitoring system that relies on patients and health care providers to report adverse effects of vaccines. The reports are considered preliminary. Most people who develop the condition recover. The F.D.A. has concluded that the benefits of the vaccine in preventing severe disease or death from the coronavirus still very much outweigh any danger, but it plans to include the proviso in fact sheets about the drug for providers and patients. “It’s not surprising to find these types of adverse events associated with vaccination,” said Dr. Luciana Borio, a former acting chief scientist at the F.D.A. under President Barack Obama. The data collected so far by the F.D.A., she added, suggested that the vaccine’s benefits “continue to vastly outweigh the risks.” In a statement released Monday, the Centers for Disease Control and Prevention said the cases have largely been reported about two weeks after vaccination and mostly in males, many aged 50 years and older. The database reports indicate that symptoms of Guillain-Barré developed within about three weeks of vaccination. One recipient, a 57-year-old man from Delaware who had suffered both a heart attack and a stroke within the last four years, died in early April after he was vaccinated and developed Guillain-Barré syndrome, according to a report filed to the database. The Biden administration is expected to announce the new warning as early as Tuesday. European regulators may soon follow suit. No link has been found between Guillain-Barré syndrome and the coronavirus vaccines developed by Pfizer-BioNTech or Moderna, the other two federally authorized manufacturers. Those rely on a different technology. Nearly 13 million people in the United States have received Johnson & Johnson’s shot, but 92 percent of Americans who have been fully vaccinated received shots developed by Pfizer-BioNTech or Moderna. Even though it requires only one dose, Johnson & Johnson’s vaccine has been marginalized by manufacturing delays and a 10-day pause while investigators studied whether it was linked to a rare but serious blood clotting disorder in women. That investigation also resulted in a warning added to the fact sheet. The new safety concern comes at a precipitous moment in the nation’s fight against Covid-19. The pace of vaccinations has slowed considerably just as a new, more contagious variant called Delta is spreading fast in under-vaccinated areas. Federal health officials are worried that the news could make some people even more hesitant to accept the vaccines developed by Pfizer-BioNTech or Moderna, even though well over 100 million people have received those vaccines, according to the Centers for Disease Control and Prevention. Almost one-third of the nation’s adults remain unvaccinated. The Biden administration has shifted away from relying on mass vaccination sites and is now enlisting community workers in door-to-door campaigns, supplying doses to primary care doctors and expanding mobile clinics in an attempt to convince the unvaccinated to accept shots. Johnson & Johnson’s vaccine has played a minor role in the nation’s inoculation campaign partly because the Baltimore plant that was supposed to supply most of the doses to the United States has been shut down for three months because of regulatory violations. The factory, operated by Emergent BioSolutions, a subcontractor, has been forced to throw out the equivalent of 75 million doses because of suspected contamination, severely delaying deliveries to the federal government. Demand for the shot also plummeted after the April safety pause. At that time, 15 women in the United States and Europe who had received the Johnson & Johnson shot had been diagnosed with the disorder. Three had died. Regulators ultimately decided that the risk was remote and far outweighed by the benefits. They attached a warning to the drug and cleared it for use, but state officials have said that the perception that the vaccine might be unsafe hurt it. Alex Gorsky, Johnson & Johnson’s chief executive, said last month that he was still hopeful that the vaccine, which has been used in 27 countries so far, would help contain the pandemic overseas. The company has promised up to 400 million doses to the African Union. Separately, Covax, the global vaccine-sharing program, is supposed to receive hundreds of millions of doses. Studies have shown that the Johnson & Johnson shot protects people against more contagious virus variants, including the Delta variant, and is highly effective at preventing severe Covid-19, hospitalizations and death. The F.D.A. shares jurisdiction over vaccines with the Centers for Disease Control and Prevention but is responsible for issuing product warnings. The Guillain-Barré cases are expected to be discussed in an upcoming meeting of a committee of outside experts who advise the C.D.C. The F.D.A. has also attached a warning to the Pfizer-BioNTech and Moderna vaccines, but some health officials described that as less serious than the warnings about Johnson & Johnson. Last month, the agency warned about an increased risk of inflammation of the heart or the tissue surrounding it — diseases known as myocarditis and pericarditis — particularly among adolescents and young adults who had received Pfizer-BioNTech or Moderna shots. But the C.D.C. said in most cases, symptoms promptly improved after simple rest or medication. The Guillian-Barré syndrome is more likely to result in medical intervention, officials said. It occurs when the immune system damages nerve cells, causing muscle weakness and occasional paralysis, according to the F.D.A. Several thousand people — or roughly 10 out of every one million residents — develop the condition every year in the United States. Most fully recover from even the most severe symptoms, but in rare cases patients can suffer near-total paralysis. The suspected cases were reported in the Vaccine Adverse Event Reporting System, or VAERS, a 30-year-old federal monitoring system. So far, researchers have not identified any particular demographic pattern, but many of the reports in the publicly available database indicate that the patients were hospitalized. Guillain-Barré syndrome has also been linked to other vaccines. The Centers for Disease Control and Prevention has said that flu vaccines, including the 1976 swine flu vaccine, led to a small increased risk of contracting the syndrome, although some studies suggested that people are more likely to develop Guillain-Barré from the flu itself than from flu vaccines. Earlier this year, the F.D.A. warned that GlaxoSmithKline’s shingles vaccine, Shingrix, could also increase the risk of the disease. Only about five million people in the U.S. have taken Johnson & Johnson’s shot since the April pause was lifted. Millions of doses that have been distributed by the federal government are sitting unused and will expire this summer....

|
Scooped by
Juan Lama
|
With concerns surrounding the Delta coronavirus variant rising globally, how effective are the current vaccines in the U.S. at protecting against the new version of the virus? According to medical experts, the three vaccines currently available each offer protection. Here’s a breakdown of each vaccine and what you should know: How effective are the COVID vaccines overall? In clinical trials, Moderna’s In clinical trials, Moderna's vaccine reported 94.1% effectiveness at preventing COVID-19 in people who received both doses. The Pfizer-BioNTech vaccine was said to be 95% effective. A new CDC study reported that a single dose of Pfizer's or Moderna's COVID vaccine was 80% effective in preventing infections. That number jumped to 90% two weeks after the second dose, the study on vaccinated health care workers showed. "These findings indicate that authorized mRNA COVID-19 vaccines are effective for preventing SARS-CoV-2 infection, regardless of symptom status, among working-age adults in real-world conditions," the U.S. agency wrote in the study. "COVID-19 vaccination is recommended for all eligible persons." Pfizer's vaccine, the only one currently authorized for use in children as young as 12, also showed heightened effectiveness among adolescents. Pfizer in late March released preliminary results from a vaccine study of 2,260 U.S. volunteers ages 12 to 15, showing there were no cases of COVID-19 among fully vaccinated adolescents compared with 18 among those given dummy shots. More intriguing, researchers found the kids developed higher levels of virus-fighting antibodies than earlier studies measured in young adults. The FDA said J&J’s vaccine offers strong protection against what matters most: serious illness, hospitalizations and death. One dose was 85% protective against the most severe COVID-19 illness, in a massive study that spanned three continents — protection that remained strong even in countries such as South Africa, where the variants of most concern were spreading at the time. The CDC reports J&J/Janssen vaccine was 66.3% effective in clinical trials at preventing COVID-19 illness in people who had no evidence of prior infection 2 weeks after receiving the vaccine. "The vaccine had high efficacy at preventing hospitalization and death in people who did get sick," the CDC notes. "No one who got COVID-19 at least four weeks after receiving the J&J/Janssen vaccine had to be hospitalized." It is not known if any of the three vaccines prevent the spread of the virus by people who are asymptomatic, though the CDC noted that "early evidence suggests that the J&J/Janssen vaccine might provide protection against asymptomatic infection." How effective are the vaccines against the new Delta variant? Data surrounding vaccine effectiveness with the Delta variant is so far limited. While studies have shown that the available vaccines work against variants, including the Delta variant, all two-dose vaccines offer significantly more protection following their second dose. Researchers in England studied how effective the two-dose AstraZeneca and Pfizer-BioNTech vaccines were against it, compared with the Alpha variant that was first detected in the U.K. The vaccines were protective for those who got both doses but were less so among those who got one dose. One recent study showed the Pfizer vaccine was 84% effective against the variant after two doses, but only 34% effective after the first dose. Moderna also announced Tuesday that a new study showed its vaccine also produced promising protection in a lab setting against the Delta variant and others currently circulating. “As we seek to defeat the pandemic, it is imperative that we are proactive as the virus evolves. We remain committed to studying emerging variants, generating data and sharing it as it becomes available. These new data are encouraging and reinforce our belief that the Moderna COVID-19 Vaccine should remain protective against newly detected variants,” Stéphane Bancel, chief executive officer of Moderna, said in a statement. Currently, little data has been released showing just how effective the Johnson & Johnson is at protecting against the Delta variant, though it is believed that the single-shot vaccine does offer protection against the variant. Dr. Scott Gottlieb, former Food and Drug Administration commissioner, reportedly said the Johnson & Johnson vaccine appears to be about 60% effective against the Delta variant. Still, medical experts say any of the three vaccines currently being used in the U.S. continue to show good results as far as protection. ”This will protect them against getting very sick and being hospitalized and even dying from the Delta variant,” Dr. Katherine Gergen-Barnett of Boston Medical Center recently told NBC10 Boston. Will a booster shot be needed? So far, there has been no recommendation from the Centers for Disease Control and Prevention surrounding booster shots with the Delta variant. Still, health experts have repeatedly cautioned that COVID-19 booster shots could be needed for fully vaccinated people, particularly as new variants spread. White House chief advisor Dr. Anthony Fauci said during an interview with MSNBC's Medhi Hasan in April that people may need to get booster shots in a year. Pfizer CEO Albert Bourla also previously said people will "likely" need a third dose within 12 months of getting fully vaccinated. So far, studies suggest that the vaccines currently in use can recognize the emerging variants — but they may not provide quite as much protection against the new strains. Boosters and new versions of vaccines that target the variants are already being explored. Pfizer-BioNTech was previously testing a third booster shot of its vaccine on fully vaccinated people. "The flexibility of our proprietary mRNA vaccine platform allows us to technically develop booster vaccines within weeks, if needed," Sahin said in a release in February. Moderna was also testing a potential third dose of its current vaccine, and a possible booster shot specifically targeting the South Africa variant. Citing early data, the company recently said the booster vaccine generated a promising immune response against the B.1.351 and P.1 variants first identified in South Africa and Brazil, respectively. Meanwhile, Johnson & Johnson CEO Alex Gorsky said during an interview with CNBC's "Squawk Box" in March that the company is well-positioned to adapt its vaccine for variants, and is working on developing software that will "help address some of these new and emerging variants."
|

|
Scooped by
Juan Lama
|
The pharma, once a frontrunner in the race to develop the first vaccine for the respiratory virus, said it will end a 23,000-person study of its experimental shot amid a restructuring of its infectious disease division. Dive Brief: - Johnson & Johnson will stop developing its experimental vaccine for respiratory syncytial virus in an unexpected retreat from a high-profile research effort that had put the pharmaceutical giant among the leading companies seeking to win the first approval of a preventive shot.
- The company said Wednesday it will discontinue a 23,000-person Phase 3 trial, called Evergreen, of its RSV vaccine in adults following a review of its drug pipeline. The company does not plan to develop the shot for pregnant women or infants, a spokesperson confirmed.
- J&J’s pullback comes amid a restructuring of its infectious disease division, which was reported by Fierce Pharma in February. Its decision also thins the RSV vaccine competition, leaving GSK and Pfizer in the lead with shots that are currently under review by the Food and Drug Administration. Moderna is also developing an RSV vaccine and could file for approval this year.
Dive Insight: J&J’s decision to halt work on its RSV vaccine extends a series of setbacks for the company in infectious disease. In January, the company halted a large trial of an HIV vaccine after disappointing results, and last month, a partner company said J&J was dialing back its hepatitis B research amid the unit’s restructuring. The company successfully developed an effective vaccine for COVID-19, but concerns over rare side effects limited its use in the U.S., where it became a distant third choice after vaccines from Pfizer and Moderna. “The decision to discontinue the Company’s RSV adult vaccine program is part of a broader effort to make strategic choices for its pipeline and research and development investments to focus on medicines with the greatest potential benefit to patients,” J&J said in its statement Wednesday. J&J began the Evergreen trial in 2021 after data from a mid-stage study showed vaccination could prevent complications from RSV infection in older adults. The Phase 3 trial, which spanned more than 300 sites across 15 countries, made J&J one of the frontrunners in development of an RSV vaccine. No results have been made public from the study, but J&J would have had a high bar to clear the marks set by RSV vaccines from GSK and Pfizer. GSK’s shot, called Arexvy, proved 83% effective in preventing cases of lower respiratory infection caused by RSV infection when compared to a placebo in a Phase 3 study of older adults. Pfizer’s was 67% effective against moderate disease in that company’s late-stage trial. Both vaccines recently won the backing of an FDA panel, making approvals by the FDA later this spring more likely. (FDA advisers did express some concern around safety, particularly for Pfizer’s.) J&J will share data from the Evergreen study with the scientific community in the future, the spokesperson said. RSV infections are usually mild in healthy adults. But the disease can be more deadly in infants and older adults. Between 6,000 to 10,000 deaths occur from RSV in older adults each year in the U.S., according to data cited by the Centers for Disease Control and Prevention. Like other pharmas, J&J has shifted its focus more heavily to cancer and immune disease drugs, which it expects will help it reach a target of $60 billion in pharmaceutical sales by 2025. While J&J’s infectious disease unit sells several HIV treatments, it earns relatively little from vaccines. The company’s COVID vaccine generated sales of $120 million in the U.S. and $2 billion internationally last year. But in the fourth quarter, J&J reported no U.S. sales and incurred $821 million in costs related to scaling back its manufacturing of the shot. Wednesday’s announcement comes four years after another RSV setback for J&J. The pharma took a $900 million write-off for an RSV drug it had obtained as part of a $1.75 billion purchase of Alios BioPharma in 2014. The accounting charge followed the company’s decision to halt mid-stage clinical work on the drug. Ned Pagliarulo contributed reporting. Editor’s note: This story has been updated with additional detail throughout.

|
Scooped by
Juan Lama
|
Comparison of the immune response to four prominent COVID-19 vaccines is among the most thorough so far, authors say. A rare head-to-head comparison shows that the COVID-19 vaccines made by Pfizer and Moderna outperform those from Johnson & Johnson and Novavax1. The data also provide a finely detailed picture of the immune protection that each vaccine offers — information that could be useful for designing future vaccines. The research was posted on the preprint server bioRxiv on 21 March. It has not yet been peer reviewed. The study assessed the 4 vaccines using 14 metrics, including levels of several types of immune cell such as T cells and B cells, as well as immune molecules called neutralizing antibodies. Such investigations are sorely needed to sort through the flood of COVID-19 vaccines in the research pipeline and on the market, researchers say. “It’s a really nice analysis by premier immunologists that builds upon what has been previously shown,” says Robert Seder, an immunologist at the US National Institute of Allergy and Infectious Diseases in Bethesda, Maryland. Previous comparisons of COVID-19 vaccines have often brought together data from different studies, which might have been conducted with slightly varying laboratory techniques. For the latest study, by contrast, researchers applied the same techniques across all the vaccines they investigated. “When you try to compare [vaccine data] between different papers, which is what many of us have been doing for over a year now, you get apples to oranges comparisons, and you can be way off,” says Shane Crotty, an immunologist at La Jolla Institute for Immunology in California and a co-author of the preprint. The four vaccines that Crotty and his co-authors examined fall into three classes. The jabs made by Moderna in Cambridge, Massachusetts, and by Pfizer in New York City and BioNTech in Mainz, Germany, are both based on messenger RNA. Johnson & Johnson (J&J) of New Brunswick, New Jersey, has produced a ‘viral vector’ vaccine that uses a harmless virus to deliver SARS-CoV-2 genetic material into host cells. The vaccine made by Novavax in Gaithersburg, Maryland, contains pieces of the SARS-CoV-2 spike protein. Strengths and weaknesses Antibody levels induced by two doses of Pfizer’s or Moderna’s mRNA vaccine tended to wane substantially over six months. By contrast, antibody levels from J&J’s one-shot vaccine were stable or even increased over time. But antibody levels measured six months after vaccination with the J&J jab were still lower than those observed six months after vaccination with an mRNA vaccine. Novavax’s two-shot regimen induced antibody responses on a par with those to the mRNA vaccines. However, after the Novavax jab, levels of CD8+ T cells, which destroy infected cells, were low to undetectable, whereas the other three vaccines performed well in this metric. These results generally support the findings of previous studies. But the latest research offers a more extensive analysis of the immune system’s response than do earlier studies, and uses an apples-to-apples approach. No losers “This is not meant to proclaim winners and losers,” says study co-author Alessandro Sette, an immunologist at La Jolla. Instead, the study is meant to “provide a comprehensive evaluation of the different variables”, he says. Novavax has received authorization for its vaccine in 38 countries. The vaccines made by Moderna, Pfizer and J&J have all received wide authorization globally. One caveat is that the study looked at the effects of the Novavax jab in only 12 people. It examined the other three vaccines in 30 volunteers each. Seder notes that the analysis considers the effects of only a two-dose regimen of the mRNA vaccines. It does not consider the protection provided by boosters, because the authors began the work in late 2020, before a third shot was recommended by health authorities. Crotty, Sette and their colleagues are now conducting a similar head-to-head study that includes mRNA boosters. Cited research available at bioRxiv (March 21, 2022): https://doi.org/10.1101/2022.03.18.484953

|
Scooped by
Juan Lama
|
BACKGROUND The Ad26.COV2.S vaccine was highly effective against severe–critical coronavirus disease 2019 (Covid-19), hospitalization, and death in the primary phase 3 efficacy analysis. METHODS We conducted the final analysis in the double-blind phase of our multinational, randomized, placebo-controlled trial, in which adults were assigned in a 1:1 ratio to receive single-dose Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe–critical Covid-19 with onset at least 14 days after administration and at least 28 days after administration in the per-protocol population. Safety and key secondary and exploratory end points were also assessed. RESULTS Median follow-up in this analysis was 4 months; 8940 participants had at least 6 months of follow-up. In the per-protocol population (39,185 participants), vaccine efficacy against moderate to severe–critical Covid-19 at least 14 days after administration was 56.3% (95% confidence interval [CI], 51.3 to 60.8; 484 cases in the vaccine group vs. 1067 in the placebo group); at least 28 days after administration, vaccine efficacy was 52.9% (95% CI, 47.1 to 58.1; 433 cases in the vaccine group vs. 883 in the placebo group). Efficacy in the United States, primarily against the reference strain (B.1.D614G) and the B.1.1.7 (alpha) variant, was 69.7% (95% CI, 60.7 to 76.9); efficacy was reduced elsewhere against the P.1 (gamma), C.37 (lambda), and B.1.621 (mu) variants. Efficacy was 74.6% (95% CI, 64.7 to 82.1) against severe–critical Covid-19 (with only 4 severe–critical cases caused by the B.1.617.2 [delta] variant), 75.6% (95% CI, 54.3 to 88.0) against Covid-19 leading to medical intervention (including hospitalization), and 82.8% (95% CI, 40.5 to 96.8) against Covid-19–related death, with protection lasting 6 months or longer. Efficacy against any severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was 41.7% (95% CI, 36.3 to 46.7). Ad26.COV2.S was associated with mainly mild-to-moderate adverse events, and no new safety concerns were identified. CONCLUSIONS A single dose of Ad26.COV2.S provided 52.9% protection against moderate to severe–critical Covid-19. Protection varied according to variant; higher protection was observed against severe Covid-19, medical intervention, and death than against other end points and lasted for 6 months or longer. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722. opens in new tab.) Published in NEJM (Feb. 9, 2022): https://doi.org/10.1056/NEJMoa2117608

|
Scooped by
Juan Lama
|
Producers of the world's top COVID-19 vaccines say they are moving quickly to test their shots against the new omicron variant. They also are developing shots tailored to the new strain. Moderna said it has a three-pronged strategy to address the threat. With the new omicron strain fueling fear around the globe that the coronavirus is regaining momentum, makers of the world’s most successful vaccines are investigating whether they need to tweak their shots. Over the last few days, Moderna, Pfizer-BioNTech, Johnson & Johnson and AstraZeneca revealed plans to address the threat posed by omicron, which emerged in South Africa and recently was detected in Australia, Israel, Hong Kong and parts of Europe. On Friday, the World Health Organization classified omicron as a “variant of concern.” Each of the companies said it's testing an omicron-specific vaccine. Moderna said it could have a tweaked version of its shot ready early next year if necessary. In the case of the delta and beta variants, Moderna needed “60-90 days” to advance new candidates to clinical testing, it said in a release. “We should know about the ability of the current vaccine to provide protection in the next couple of weeks,” Moderna’s chief medical officer Paul Burton told the BBC. “The remarkable thing about the mRNA vaccines," Burton said, "is that we can move very fast.” Moderna provided the most detailed information—laying out a three-pronged strategy—on its plan to address the variant. First, with 306 participants, the biotech has begun a study of a higher-dose version of its booster to see if it provides superior protection against the strain. Secondly, Moderna is studying two multi-valent booster candidates that were designed in anticipation of mutations such as those that have appeared in the omicron variant. Lastly, the company is developing its omicron-specific shot. Moderna’s strategy is the “right one,” said (PDF) analysts at ODDO BHF, who stopped short of adjusting revenue figures for the company for 2022 and 2023. “In less than three months we could hope, on paper, to have a specific vaccine candidate against this new form and a market launch between three and six months from now,” ODDO BHF analysts wrote to investors. Meanwhile, Comirnaty partners Pfizer and BioNTech will be ready to adapt a new vaccine "within six weeks and ship initial batches within 100 days," BioNTech said in an emailed statement. The company added that there is a greater chance that solving the new strain will require a tweaked shot. "The omicron variant differs from previously observed variants because it has additional mutations located in the spike protein," BNT said. "We expect data from the laboratory tests in about two weeks. These data will provide more information about whether B.1.1.529 could be an escape variant that may require an adjustment of our vaccine." For its part, J&J has been working with academic groups in South Africa and from around the world to evaluate the effectiveness of its adenovirus vaccine versus the omicron variant, the company said in a release. “We remain confident in the robust humoral and cell-mediated immune responses elicited by the Johnson & Johnson COVID-19 vaccine, demonstrated by the durability and breadth of protection against variants to date,” Mathai Mammen, the global head of Janssen R&D, said in a statement. “In parallel, we have begun to work to design and develop a new vaccine against omicron and will rapidly progress it into clinical studies if needed.” AstraZeneca is taking the same measures—testing its current vaccine while developing another to defend against the variant. Additionally, it is testing the effectiveness of the monoclonal antibody treatment it is developing for the prevention and treatment COVID-19. "AstraZeneca has developed, in close collaboration with Oxford University, a vaccine platform that enables us to respond quickly to new variants that may emerge," the company said in an emailed statement. "AstraZeneca is also already conducting research in locations where the variant has been identified, namely in Botswana and Eswatini, that will enable us to collect real world data of Vaxzevria against this new virus variant." In a note to investors, Berenberg analysts said that the speed with which mRNA vaccines can be developed will be critical if new variant-specific shots are needed. “Due to more mutations observed in omicron, the effectiveness of the current vaccination regimen will likely be reduced, which underscores the need for boosters and potentially variant-specific vaccines,” the analysts wrote. “We believe mRNA technology is the solution to lead us out of the pandemic.”

|
Scooped by
Juan Lama
|
All three currently authorized COVID-19 vaccines still showed signs of a strong immune response eight months later without a booster, according to a study published Friday in the New England Journal of Medicine. The study analyzed specific markers of immunity found in the blood of people vaccinated with Pfizer, Moderna and the Johnson & Johnson vaccines. Echoing evidence from the real world, researchers found cellular signatures suggesting that all three vaccines produce strong and long-lasting protection from severe illness. But the analysis also hinted at differences in the way the vaccines produce antibodies -- with Pfizer and Moderna antibodies spiking and then fading quickly, while Johnson & Johnson antibodies started at a lower level but remained more stable over time. "By month eight, antibody responses were comparable for these three vaccines," said Dr. Dan Barouch, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, who coauthored the research. Pfizer and Moderna vaccines rely on the same type of technology, called mRNA, while Johnson & Johnson uses a different technology, called viral vector. The two technologies prompt different types of immune responses. Thought the pandemic, scientists have used antibodies -- virus fighting proteins in the blood -- as one indication that vaccines are working. But antibodies are only one part of the body's overall immune response. This new study is among the first to directly compare not just antibodies, but also T-cells, across all three vaccines. T-cells are also a crucial part of the immune system, and may offer longer-lasting protection even after antibodies fade. "We think the antibodies are often more relevant preventing against infection, and the T-cells are more relevant killing the virus -- so preventing severe disease," said Dr. Todd Ellerin, director of infectious diseases at South Shore Health and an ABC News medical contributor. "T-cell responses likely contribute to vaccine protection against severe disease," said Barouch. "T-cell responses were relatively stable for all three vaccines for eight months." This new study is among the first to directly compare not just antibodies, but also T-cells, across all three vaccines. T-cells are also a crucial part of the immune system, and may offer longer-lasting protection even after antibodies fade. "We think the antibodies are often more relevant preventing against infection, and the T-cells are more relevant killing the virus -- so preventing severe disease," said Dr. Todd Ellerin, director of infectious diseases at South Shore Health and an ABC News medical contributor. "T-cell responses likely contribute to vaccine protection against severe disease," said Barouch. "T-cell responses were relatively stable for all three vaccines for eight months." Original findings published in NEJM (October 15, 2021): https://doi.org/10.1056/NEJMc2115596

|
Scooped by
Juan Lama
|
Background: While Coronavirus disease 2019 (Covid-19) vaccines are highly effective, breakthrough infections are occurring. Booster vaccinations have recently received emergency use authorization (EUA) for certain populations but are restricted to homologous mRNA vaccines. We evaluated homologous and heterologous booster vaccination in persons who had received an EUA Covid-19 vaccine regimen. Methods: In this phase 1/2 open-label clinical trial conducted at ten U.S. sites, adults who received one of three EUA Covid-19 vaccines at least 12 weeks prior to enrollment and had no reported history of SARS-CoV-2 infection received a booster injection with one of three vaccines (Moderna mRNA-1273 100-mcg, Janssen Ad26.COV2.S 5x1010 virus particles, or Pfizer-BioNTech BNT162b2 30-mcg; nine combinations). The primary outcomes were safety, reactogenicity, and humoral immunogenicity on study days 15 and 29. Results: 458 individuals were enrolled: 154 received mRNA-1273, 150 received Ad26.CoV2.S, and 154 received BNT162b2 booster vaccines. Reactogenicity was similar to that reported for the primary series. Injection site pain, malaise, headache, and myalgia occurred in more than half the participants. Booster vaccines increased the neutralizing activity against a D614G pseudovirus (4.2-76-fold) and binding antibody titers (4.6-56-fold) for all combinations; homologous boost increased neutralizing antibody titers 4.2-20-fold whereas heterologous boost increased titers 6.2-76-fold. Day 15 neutralizing and binding antibody titers varied by 28.7-fold and 20.9-fold, respectively, across the nine prime-boost combinations. Conclusion: Homologous and heterologous booster vaccinations were well-tolerated and immunogenic in adults who completed a primary Covid-19 vaccine regimen at least 12 weeks earlier. Available as preprint in medRxiv (Oct. 13, 2021): https://doi.org/10.1101/2021.10.10.21264827

|
Scooped by
Juan Lama
|
A two-dose version of Johnson & Johnson's coronavirus vaccine provides 94% protection against symptomatic infection, the company said Tuesday -- making a two-dose regimen of J&J's Janssen vaccine comparable to a two-dose regimen of Moderna's or Pfizer's. Plus, the company said, adding a booster dose to a single shot of the vaccine raised immunity even more, and should also protect people strongly against infection. The company released some details of three studies looking at various aspects of its Janssen vaccine, and said that, taken together, they showed the vaccine provided long-lasting protection that could be boosted with an extra shot. "Our large real-world-evidence and Phase 3 studies confirm that the single-shot Johnson & Johnson vaccine provides strong and long-lasting protection against COVID-19-related hospitalizations," Dr. Mathai Mammen, global head of Janssen Research & Development, said in a statement. "Our single-shot vaccine generates strong immune responses and long-lasting immune memory. And, when a booster of the Johnson & Johnson COVID-19 vaccine is given, the strength of protection against COVID-19 further increases." Johnson & Johnson's single-dose vaccine was given emergency use authorization by the US Food and Drug Administration on February 27. It has been given to about 14.8 million Americans, according to the US Centers for Disease Control and Prevention. The company's ongoing Phase 2 trial of a two-dose regimen showed giving two doses 56 days apart provided 100% protection against severe Covid-19 and 94% protection against moderate to severe Covid-19 in the United States. Globally, the two-dose regimen provided 75% protection against moderate-to-severe Covid-19, the company said. A second study showed people given a booster shot six months or longer after their first dose had a 12-fold increase in antibodies -- compared to a four-fold increase for people who got a second dose at two months. So protection should be stronger if people get boosters later, Dr. Dan Barouch, head of Beth Israel Deaconess' Center for Virology and Vaccine Research, told CNN. "If you wait longer and have boost at six months or later then you likely will have better boost," said Barouch. Third, the company said a real-world evidence study of 390,000 people in the US, using health insurance records through July -- so covering the Delta variant -- showed the one-shot J&J vaccine was 81% effective at preventing hospitalizations. "The Johnson & Johnson single-shot COVID-19 vaccine showed vaccine effectiveness against COVID-19-related hospitalizations at 86% for participants younger than 60 years, and 78% for those 60 years and older," the company said. "Among 390,517 vaccinated and 1,524,153 matched unvaccinated individuals, vaccine effectiveness 79% for COVID-19 and 81% for COVID-19-related hospitalizations," the Janssen-led research team wrote in a study posted online in a preprint. "In high-Delta-incidence states, rates of observed COVID-19 were higher in both groups than in the national cohort," they added. "In these states, vaccine effectiveness for observed COVID-19 was 79% overall and 78% during June and July, the months where Delta variant incidence was highest," they added. Barouch, who has worked with Janssen to test the vaccine but who was not directly involved in the three studies, said people who got the Johnson & Johnson vaccine should be reassured by the data. "All the vaccines in the US have shown robust and durable protection against severe disease and hospitalization," he said. "Ultimately, the job of a vaccine is to keep you from being sick and keep you from going into the hospital and to keep you alive, and all of the vaccines are doing that." Data on the J&J vaccine has come later than data about the Moderna and Pfizer/BioNTech vaccines because J&J's was authorized around two months later. Johnson & Johnson has said it will submit all of this data to the FDA for potential consideration for adding a booster dose, and perhaps for consideration to authorize a two-dose regimen. The Janssen vaccine is made using a different technology from Moderna's and Pfizer's vaccines. They deliver messenger RNA or mRNA directly to the body wrapped in compounds called lipids. The J&J vaccine is made using an adenovirus, a common cold virus, that's been engineered so it can get into cells, but then stops. It delivers genetic instructions that way. Barouch said there is room for a variety of approaches. "A single shot gives robust and durable protection over a substantial period of time of time with minimal evidence of decline," Barouch said. "I think the single dose vaccine is a reasonable option for people and for countries that want a simple and convenient vaccine that can be administered quickly," he added. "For outstanding protection, then a second shot can be given at any time between two months and eight months -- and the longer you wait, the better." That, he said, is because the body mounts a variety of immune responses. Antibodies -- immune system proteins that can either flag an invader or directly attack and neutralize it -- build up quickly but can wane over time. The body also produces cells called B cells and T cells, and these contribute to longer-term protection. Stimulating B cells with a boost after time -- after they have become less active -- appears to cause them to generate fresh antibodies more effectively, he said. Barouch said the J&J vaccine may appear less effective in countries outside the United States because it was tested in many countries when variants were circulating that can evade the protection offered by vaccines. The Beta or B.1.351 variant is an example -- it has so-called escape mutations that help it hide from the immune response. It circulated widely in South Africa but has been outcompeted in the US by Delta, which does not appear to escape immune protection as well. Johnson & Johnson Press Release (Sept. 21, 2021):

|
Scooped by
Juan Lama
|
Clinical trial data find an Ebola vaccine regimen safe, well tolerated, and produces a strong immune response in people over the age of one. Welcome news for Ebola virus disease prevention was published yesterday in two papers presenting data showing that Johnson & Johnson’s (J&J) two-dose Ebola vaccine regimen is safe, well tolerated, and produces a strong immune response in people over the age of one. This news comes five years after the largest outbreak of Ebola since its discovery and just one month after an Ebola outbreak was declared in the Ivory Coast. The study aimed to assess the safety and long-term immunogenicity of a two-dose vaccine regimen. The first vaccine is the adenovirus type 26 vector-based vaccine encoding the Ebola virus glycoprotein (Ad26.ZEBOV). The second is the modified vaccinia Ankara vector-based vaccine, encoding glycoproteins from Ebola virus, Sudan virus, and Marburg virus, and the nucleoprotein from the Tai Forest virus (MVA-BN-Filo). The authors found that the vaccine regimen was well tolerated and induced antibody responses to Zaire ebolavirus 21 days after the second dose in 98% of participants, with the immune responses persisting in adults for at least two years. Conducted in Sierra Leone, the EBOVAC-Salone study is the first to assess the safety and tolerability of this vaccine regimen in a region affected by the 2014–2016 West African Ebola outbreak, which was the worst on record. It is also the first study reporting the evaluation of this vaccine regimen in children. The data were published in The Lancet Infectious Diseases in two separate papers: “Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: a randomized, double-blind, controlled trial” and “Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomized stage 1, and a randomized, double-blind, controlled stage 2 trial.” During the 2014–16 outbreak of Ebola in West Africa, 28,652 cases and 11,325 deaths from Ebola were reported. Approximately 20% of cases were in children under 15 years, and children younger than five years are at a higher risk of death than adults. “This study represents important progress in the development of an Ebola virus disease vaccine regimen for children, and contributes to the public health preparedness and response for Ebola outbreaks,” said Muhammed Afolabi, MD, assistant professor at the London School of Hygiene and Tropical Medicine. The clinical trial recruited participants from September 2015 to July 2018. The study was divided into two stages. In stage one, 43 adults aged 18 years or older received the Ad26.ZEBOV vaccine followed by the MVA-BN-Filo vaccine after 56 days. In stage two, 400 adults and 576 children and adolescents (192 in each of the three age cohorts of 1–3, 4–11 and 12–17 years of age) were vaccinated with either the Ebola vaccine regimen (Ad26.ZEBOV followed by MVA-BN-Filo) or a single dose of a meningococcal quadrivalent conjugate vaccine followed by placebo on day 57. Adults participating in stage one of the study were offered a booster dose of A26.ZEBOV two years after the first dose which induced a strong immune response within seven days. “To protect people from Ebola, we will need a range of effective interventions,” said Daniela Manno, clinical assistant professor at the London School of Hygiene and Tropical Medicine. “These findings support the additional strategy of providing an Ad26.ZEBOV booster to previously immunized individuals at the start of an Ebola virus disease outbreak.” The study findings have already contributed to the approval and marketing authorization of the two-dose Ebola vaccine regimen in July 2020 by the European Medicines Agency, for use in both children and adults. It also contributed to the WHO Prequalification in April 2021, which will facilitate formal registrations of this vaccine regimen in countries at risk of Ebola virus disease outbreaks. “The threat of future Ebola virus disease outbreaks is real and it’s important to remember that this disease has definitely not gone away,” noted Deborah Watson-Jones, PhD, clinical epidemiologist from the London School of Hygiene & Tropical Medicine. “Despite the additional global challenges around COVID-19, we must not slow down efforts to find effective ways of preventing Ebola virus epidemics and, should outbreaks occur, of containing them rapidly. Vaccines have a key role in meeting both of these objectives.” In May 2021, J&J said it would donate thousands of Ebola vaccine regimens in support of a WHO early access clinical program launched in response to an outbreak in Guinea and aimed at preventing Ebola in West Africa. The program began by vaccinating health workers, other frontline workers and others at increased risk of exposure to the Ebola virus in Sierra Leone. To date, more than 250,000 individuals participating in clinical trials and vaccination initiatives have received at least the first dose of the J&J Ebola vaccine regimen, including 200,000 who have been fully vaccinated. Further studies are being carried out in Sierra Leone to investigate whether the vaccines are safe and induce immune responses among infants aged under one year, and to follow up the adult and child participants over five years to assess the potential for long term protection. “This is an example of crucial research which brings together scientists from Africa with partners in the north and pharmaceutical companies to tackle a major public heath threat in Africa,” said Sir Brian Greenwood, MD, professor of clinical tropical medicine, London School of Hygiene and Tropical Medicine. The research in Kambia district was a collaboration between the London School of Hygiene & Tropical Medicine and Sierra Leone’s College of Medicine and Allied Health Sciences under the EBOVAC1 project. See research published in The Lancet Infectious Diseases (Sept.13, 2021): https://doi.org/10.1016/S1473-3099(21)00128-6 https://doi.org/10.1016/S1473-3099(21)00125-0

|
Scooped by
Juan Lama
|
People who received a booster six to eight months after their initial Johnson & Johnson shots saw antibodies increase nine-fold higher than 28 days after the first shot, the comapany said. The data comes from two Phase 2 studies conducted in the United States and Europe, the company said in a statement. Some of the 2,000 or so people in the studies got booster doses six months after their first doses of J&J's Janssen vaccine. "New interim data from these studies demonstrate that a booster dose of the Johnson & Johnson COVID-19 vaccine generated a rapid and robust increase in spike-binding antibodies, nine-fold higher than 28 days after the primary single-dose vaccination," the company said in its statement. "We have established that a single shot of our COVID-19 vaccine generates strong and robust immune responses that are durable and persistent through eight months. With these new data, we also see that a booster dose of the Johnson & Johnson COVID-19 vaccine further increases antibody responses among study participants who had previously received our vaccine," Dr. Mathai Mammen, global head of research and development for Janssen, said in a statement. J&J said it was in discussions with the US Food and Drug Administration, US Centers for Disease Control and Prevention, European Medicines Agency, World Health Organization and other health authorities about the need for offering a booster dose of the Janssen vaccine. "We look forward to discussing with public health officials a potential strategy for our Johnson & Johnson COVID-19 vaccine, boosting eight months or longer after the primary single-dose vaccination," Mammen added. Many people who received the J&J vaccine have been clamoring for information about whether they will need a booster shot. US federal government officials have said they are preparing to start offering a booster dose to people who got Moderna's or Pfizer's Covid-19 vaccine after data showed boosters can amp up the antibody response -- and after studies started showing an uptick in infections in both vaccinated and unvaccinated people. The more transmissible Delta variant is partly to blame, experts say, as is a waning immune response. The Janssen vaccine was authorized at the end of February, more than two months after Moderna's and Pfizer's vaccines were authorized. About 14 million Americans have received the J&J vaccine, according to the CDC. Dr. Dan Barouch, a vaccine researcher at Beth Israel Deaconess Medical Center and Harvard Medical School who is not involved in the two clinical studies but is helping study J&J vaccines, said the findings support getting a booster shot, but only after a delay. "The boost at six months is going to look very impressive and substantially greater than what has already been reported in terms of the two month boost, and that is significant because it, in my opinion, the boost should not be at two months, but it really should be at six months or later," Barouch told CNN. Neither of the studies looked at real-world efficacy, so the company has not demonstrated that people who get boosters will be less likely to become infected or to develop severe disease. But researchers are beginning to agree that antibody levels do indicate immune protection. The Johnson & Johnson vaccine is made differently from Pfizer's and Moderna's. Those two vaccines use messenger RNA or mRNA, encased in little lipid particles, to carry instructions to the body to start an immune response. The Janssen vaccine uses a crippled common cold virus called an adenovirus to carry in similar instructions. There had been worries that a booster dose of such a viral vector vaccine might not work effectively because of the possibility the body would generate an immune response against the vector, also. "There was a theoretical concern that the generation of anti-vector antibodies by the first shot could impede the use of it again," Barouch said. "I think these data put that to rest." Federal health officials have said they believe a booster dose of the Janssen vaccine will be needed at some point. "I'm quite certain that the FDA, CDC, NIH, White House will use these data to likely justify or recommend a booster for J&J-vaccinated people, probably with a second shot of J&J," Barouch said. Janssen's Press Release (August 25, 2021):

|
Scooped by
Juan Lama
|
The Janssen COVID-19 vaccine has become the fifth vaccine against the pandemic to be granted emergency use approval. Union health minister Mansukh Mandaviya tweeted today to inform that the single-dose vaccine manufactured by American pharmaceutical giant Johnson & Johnson has been given approval for Emergency Use in India. The development comes just two days after the company had applied for Emergency Use Authorization in India. The company had said in a statement, “On August 5, 2021 Johnson & Johnson Pvt Ltd applied for EUA of its single-dose Covid-19 vaccine to the government of India.” They had submitted the application based on topline efficacy and safety data from the Phase 3 Ensemble clinical trial. The trial had shown that the single-dose vaccine is 85% effective in preventing severe Covid-19 disease across all regions studied. It had also shown protection against Covid-19 related hospitalisation and death, which begins 28 days after vaccination. Data from a clinical trial in South Africa also suggest that the vaccine is effective in preventing severe illness and death from the Delta and Beta variants of the coronavirus. The company has tied up with Biological E for the supply of the vaccine in India. The vaccine will also be produced in India, at Biological E plant in Hyderabad. “Johnson & Johnson is working with Biological E. Limited on the manufacturing of the Johnson Covid-19 vaccine. We believe Biological E. will be an important part of our global Covid-19 vaccine supply network, where multiple manufacturing sites are involved in the production of our vaccine across different facilities, sometimes in different countries and continents, before the vaccine can be distributed”, an official statement released by Johnson and Johnson had earlier said. The vaccine developed by Janssen Vaccines in Leiden, Netherlands, a subsidiary of J&J, is a viral vector vaccine. It is based on a human adenovirus that has been modified to contain the gene for making the spike protein of the virus that causes COVID-19. Responding to this spike protein, the immune system in the body produces antibodies that fights the virus. The four vaccines that India has already approved are Covaxin developed by Astra Zeneca and Oxford University, Covishield developed by Bharat Biotech, Sputnik V developed in Russia and the vaccine developed by Moderna in the USA.

|
Scooped by
Juan Lama
|
Johnson & Johnson’s Covid-19 vaccine may trigger a rare neurological condition in a small number of people who receive the vaccine, the Food and Drug Administration said Monday. Reports to a database operated jointly by the agency and the Centers for Disease Prevention and Control suggest there may be a link between the inoculations and Guillain-Barré syndrome, a form of progressive paralysis that is generally reversible, the FDA said in a statement. The agency said there have been about 100 preliminary reports of GBS, as the condition is often called, in people who have received the J&J vaccine. To date, about 12.8 million doses of the J&J vaccine have been used in the United States, suggesting a rate of about one case of GBS per 128,000 people vaccinated. In most cases, the people reporting the condition developed it about two weeks after receiving the one-dose vaccine. Most are older males, which fits with the known pattern of GBS, said John Moore, an immunologist at Weill Cornell Medicine. “A rare, but very probably real consequence of the vaccine,” said Paul Offit, a vaccine expert from Children’s Hospital of Philadelphia. “Again, the benefits of the vaccine clearly and definitely outweigh its very rare risks.” The FDA’s statement comes after a similar signal of GBS was identified with AstraZeneca’s Covid vaccine. Last week the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee recommended adding a warning to the label of the AstraZeneca vaccine, Vaxzevria, to inform health care providers. Its statement said it is not currently clear if the AstraZeneca vaccine triggers the condition. “At this stage the available data neither confirms nor rules out a possible association with the vaccine,” the EMA said. Both the AstraZeneca and J&J vaccines are what are known as viral-vectored vaccines — meaning they carry harmless genetic material from the SARS-CoV-2 virus into the body to trigger an immune response. The fact that both these vaccines now appear to be associated with GBS suggest it could be a class effect — something one might expect to see with other viral-vectored Covid vaccines, Offit said. The FDA said to date there has been no signal to suggest the messenger RNA vaccines — those made by Moderna or Pfizer and its partner BioNTech — trigger GBS. There are multiple known causes of Guillain-Barré syndrome. Some viral infections, including influenza, have been shown to increase one’s risk of developing GBS, as have some gastrointestinal infections. And several vaccines have been shown to, on rare occasions, induce the condition. Most famously, the 1976 swine flu vaccination program in the United States was halted after a number of cases of Guillain-Barré syndrome were reported in people who received the vaccine. It was later estimated that the syndrome occurred at a rate of about one case per 100,000 doses of the swine flu vaccine. Though some flu seasons there is no signal of increased GBS cases linked to vaccination, other years there does appear to be an increase. In those cases, it has been estimated that there are one or two additional cases of GBS for every 1 million doses of vaccine given, the CDC estimates. Between 3,000 and 6,000 people in the United States develop GBS every year. The underlying cause of the condition causes nerve inflammation that can result in pain, numbness, muscle weakness, and difficulty walking.

|
Scooped by
Juan Lama
|
Johnson & Johnson said its Covid vaccine was effective against the highly contagious Delta variant, adding to the growing body of evidence that the most widely available Covid shots offer protection against its most dangerous variants. Even eight months after inoculation, the single-shot J.&J. vaccine is proving to be highly effective against Delta, the company reported on Thursday, a reassuring finding for the 11 million Americans who have gotten the shot and for countries around the world betting on receiving the vaccine. In the United States, the variant, first identified in India, now accounts for an estimated one in four new cases, and the C.D.C. has listed it in 23 states. Johnson & Johnson said its vaccine showed a small drop in potency against the Delta variant, compared with its effectiveness against the original virus, and a larger drop against the Beta variant first identified in South Africa. That is the same pattern seen with the mRNA vaccines made by Pfizer-BioNTech and Moderna. The intense discourse about Delta’s threat has left some people who are vaccinated feeling anxious about whether they are protected. The variant’s global spread has prompted new restrictions from Ireland to Malaysia. Frustration had been building about the lack of clarity around the Johnson & Johnson vaccine’s efficacy against Delta. And reports of a cluster of cases among players on the Yankees baseball team who had received the Johnson & Johnson shot, though all asymptomatic or mild, did nothing to assuage fears. Studies have shown that the Delta and Beta variants slightly lower the efficacy of the Pfizer and Moderna vaccines. For Pfizer, studies show that two doses offer 88 percent protection against the Delta variant, just below the 93 percent protection against Alpha. The Moderna vaccine has performed similarly to Pfizer’s in earlier studies. Johnson & Johnson has collected less data than its peers on the vaccines, and the study released on Thursday was small and has not yet been published in a scientific journal. Updates on the efficacy of the Johnson & Johnson vaccine have been slow because it was rolled out later than the Pfizer and Moderna vaccines in the United States. The vaccine offered about 72 percent protection against early versions of the virus. Research cited available at bioRxiv (July 1, 2021): https://www.biorxiv.org/content/10.1101/2021.07.01.450707v1
|



 Your new post is loading...
Your new post is loading...