Your new post is loading...

|
Scooped by
Juan Lama
|
Drug hunters are finding that ancient virus-like artifacts in the human genome could offer new avenues to treat neurodegeneration, cancer, autoimmunity and even aging with antibodies, vaccines and antiretroviral agents. A new wave of therapies is taking aim at virus-like elements spread throughout the human genome. These genomic parasites, which have accumulated over the course of human evolution, are embedded in the vast expanse of DNA sequences that dwell in the spaces between our genes — what scientists call the ‘dark genome’. Although they were largely ignored in the past, growing scientific evidence indicates that these normally dormant elements — including retroviruses, transposons and other repetitive sequences — can reactivate, triggering inflammation, cancer and other disease-related cellular damage. Several companies have recognized this reactivation as an untapped clinical opportunity and are developing therapeutics to stifle these ancient interlopers. For example, Transposon Therapeutics’ lead drug candidate, TPN-01, was originally devised as an antiretroviral drug to block proliferation of HIV-1, but also appears to be a potent inhibitor of a dark genome-dwelling transposon known as LINE-1 (long interspersed nuclear element-1). This February, Transposon Therapeutics announced promising results from a phase 2 trial testing TPN-01 as a treatment for the neurodegenerative disorder progressive supranuclear palsy (PSP). Venture investors and pharma are paying attention. In September 2023, dark genome startup Rome Therapeutics reported $77 million in series B funding from major players including Sanofi, Bristol Myers Squibb and Johnson & Johnson — bringing their total take for this round to $149 million. Many startups initially faced an uphill battle persuading investors of the clinical opportunities in the dark genome. “They’re tainted with the assumption of non-functionality and non-importance,” says Joseph Dukes, CSO at Oxford, UK-based Enara Bio, a company engaged in scanning the dark genome for antigens that may offer fruitful targets for cancer immunotherapy. Rosana Kapeller, CEO and co-founder of Rome Therapeutics, recalls being greeted with skepticism the first time she presented the company’s strategy at the J.P. Morgan Healthcare Conference in 2020. “People looked at me and said, ‘You’re nuts’,” she recalls. The skepticism was understandable given that, until relatively recently, drug discovery has nearly exclusively focused on the exome: the 2% of the genome that codes for protein. But upwards of half the human genome consists of repetitive dark genome elements that have accumulated throughout evolution. For example, LINE-1 has a history dating back over 100 million years, and Kapeller estimates that this element composes roughly 20% of the genome. Most of these sequences are defunct fragments, but the roughly 150 intact LINE-1 sequences can potentially proliferate via a copy-and-paste mechanism driven in part by the LINE-1-encoded reverse transcriptase (RT) enzyme. Human endogenous retroviruses (HERVs) — which retain similar protein-coding genes to those seen in retroviruses like HIV, but lack the ability to produce replicating particles — are another important target. Both LINE-1 and HERV sequences are normally maintained in a quiescent state via DNA methylation, but events that lead to demethylation can cause these stowaways to ‘wake up’, potentially triggering a powerful immune response or other pathological outcomes....

|
Scooped by
Juan Lama
|
Newswise — Retroviruses are viruses that multiply by incorporating their genes into the genome of a host cell. If the infected cell is a germ cell, the retrovirus can then be passed on to the next generation as an “endogenous” retrovirus (ERV) and spread as part of the host genome in that host species. In vertebrates, ERVs are ubiquitous and sometimes make up 10 per cent of the host genome. However, most retrovirus integrations are very old, already degraded and therefore inactive – their initial impact on host health has been minimised by millions of years of evolution. A research team led by the Leibniz Institute for Zoo and Wildlife Research (Leibniz-IZW) has now discovered a recent case of retrovirus colonisation in a rodent from New Guinea, the white-bellied mosaic-tailed rat. In a paper in the scientific journal "Proceedings of the National Academy of Sciences", they describe this new model of virus integration. The observations on this process will help to improve our understanding how retroviruses rewrite host genomes.
Retroviruses, such as the pathogen responsible for AIDS (HIV-1), integrate into the genome of the host cells they infect during their life cycle. When this happens in the germline (egg cells or cells that produce sperm) of the host, the retrovirus can actually become a gene of the host itself. This process is apparently common, as up to 10 percent of the genomes of most vertebrates consist of the remnants of such ancient infections. One of the best studied models of this process is the koala retrovirus (KoRV), which is currently colonising the koala genome. „What happens to the virus and the host during this process of genome colonisation we do not know, as most such events occurred millions of years ago and we only see the leftover ‘fossils’ of the retrovirus”, says Prof Alex Greenwood, head of the Leibniz-IZW Department of Wildlife Diseases. “Nor do we know what the host suffered health-wise during the infection process. The koala retrovirus (KoRV) is one of the few models of this process that occurs in real time and where we can observe the effects of genome colonisation on the host animal.”
There is now some evidence that KoRV-related viruses are circulating in rodents and bats in Papua New Guinea and Indonesia. A group led by Greenwood and Dr Saba Mottaghinia, former PhD student in Greenwood’s department, analysed 278 samples from seven bat and one rodent family endemic to the Australo-Papua region (Australia and New Guinea). They discovered a retrovirus that is currently colonising the genome of an endemic rodent from New Guinea, the white-bellied mosaic-tailed rat (Melomys leucogaster). This is only the second example from the Australo-Papuan region, after KoRV, of a retrovirus that has colonised a genome while retaining a functional viral life cycle. The gibbon ape leukaemia viruses (GALV), a group of viruses discovered in gibbons and woolly monkeys at a research facility in Thailand in the 1960s, are very closely related to KoRV. This is a curious and surprising relationship, as there is a geographical barrier, known as the Wallace lineage, which separates the fauna of Southeast Asia from Indonesia, Papua New Guinea and Australia's fauna. However, there is evidence that the gibbons and woolly monkeys at the research facility in Thailand have been infected with viruses from Papua New Guinea. „The discovery of GALV-like viruses in rodents and bats in Indonesian and Australian rodents and bats from New Guinea suggests that these viruses, and possibly also KoRV, originated in New Guinea”, says Greenwood, who initiated the research project funded by the German Research Foundation.
The Leibniz-IZW team, together with scientists from the Charité, the Robert Koch Institute, the Max Delbrück Center, the University of Nicosia, California State University Fullerton, the South Australian Museum and Museum Victoria, examined 278 bat and rodent samples from Australia and New Guinea for KoRV and GALV-like viruses. They detected a GALV, the Woolly Monkey Virus (WMV) in a population of the white-bellied mosaic-tailed rat, an endemic rodent from New Guinea. In five of the rats from two New Guinea collection sites, WMV was integrated into the genome at the same location, indicating that it has spread as a gene and not by infection, i.e. it has become part of the genome. However, in other white-bellied mosaic-tailed rat populations the virus was absent – similar to KoRV in koalas, where all koalas in northern Australia have KoRV in their genome, whereas there are koalas in southern Australia that do not have intact KoRV. The virus, now called the “complete Melomys woolly monkey virus” (cMWMV), was able to infect cell lines, produce new viral progeny, was visible by electron microscopy as viral particles that detached from the cell membrane, and was even sensitive to the antiretroviral drug AZT.
„The virus has all the characteristics of an exogenous infectious retrovirus, but is endogenous. It is probably a very recent colonisation event, much younger than KoRV", says Dr Saba Mottaghinia, the lead author of the paper in the „Proceedings of the National Academy of Sciences“. The results suggest that cMWMV is a new model for retroviral colonisation of the host genome that occurs in real time, as in KoRV, and they also suggest that GALVs like WMV originated in the diverse fauna of New Guinea. The discoveries in New Guinea have certainly not been exhausted. “There are hundreds of mammalian species from this region that have not yet been studied, suggesting that many more viruses and models exist in this region”, says Greenwood.
The authors dedicate the study to Ken P. Aplin of the South Australian Museum, who sadly passed away during the course of the project
Published in PNAS (Feb. 1, 2024): https://doi.org/10.1073/pnas.2220392121

|
Scooped by
Juan Lama
|
A discovery gives experts new ideas for developing vaccines to treat or even prevent cancer.

|
Scooped by
Juan Lama
|
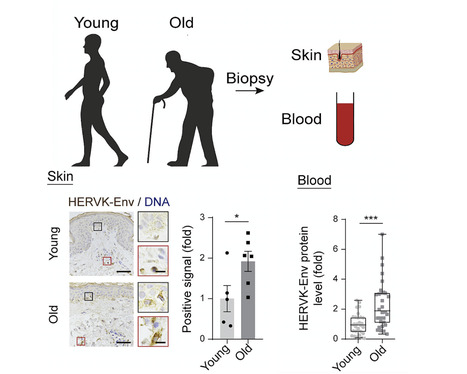
Liu and colleagues uncover the ways in which derepression of human endogenous retrovirus triggers cellular senescence and tissue aging; the findings provide fresh insights into therapeutic strategies for alleviating aging. Summary Whether and how certain transposable elements with viral origins, such as endogenous retroviruses (ERVs) dormant in our genomes, can become awakened and contribute to the aging process is largely unknown. In human senescent cells, we found that HERVK (HML-2), the most recently integrated human ERVs, are unlocked to transcribe viral genes and produce retrovirus-like particles (RVLPs). These HERVK RVLPs constitute a transmissible message to elicit senescence phenotypes in young cells, which can be blocked by neutralizing antibodies. The activation of ERVs was also observed in organs of aged primates and mice as well as in human tissues and serum from the elderly. Their repression alleviates cellular senescence and tissue degeneration and, to some extent, organismal aging. These findings indicate that the resurrection of ERVs is a hallmark and driving force of cellular senescence and tissue aging. Highlights - Derepression of the endogenous retrovirus contributes to programmed aging
- Upregulation of HERVK triggers the innate immune response and cellular senescence
- Extracellular HERVK retrovirus-like particles induce senescence in young cells
- Endogenous retrovirus serves as a potential target to alleviate aging
Published in Cell (Jan. 6, 2023): https://doi.org/10.1016/j.cell.2022.12.017

|
Scooped by
Juan Lama
|
Understanding every step in the life cycle of a virus is crucial for identifying potential targets for treatment. Now, scientists at the Institute of Science and Technology (IST) Austria were able to show how a virus from the retrovirus family—the same family as HIV—protects its genetic information and becomes infectious. Furthermore, they show an unexpected flexibility of the virus. This study is published in the journal Nature Communications. Viruses are perfect molecular machines. Their only goal is to insert their genetic material into healthy cells and thus multiply. With deadly precision, they thereby can cause diseases that cost millions of lives and keep the world on edge. One example for such a virus, although currently less discussed, is HIV that causes the ongoing global AIDS-epidemic. Despite the progress made in recent years, 690 000 people died in 2019 alone as a result of the virus infection. "If you want to know the enemy, you have to know all its friends," says Martin Obr, postdoc at the Schur group at IST Austria. Together with his colleagues, he therefore studies a virus belonging to the same family as HIV—the Rous sarcoma virus, a virus causing cancer in poultry. With its help, he now gained new insights into the important role a small molecule plays in the assembly of these type of viruses. Protecting the virus blueprint In their study, published in the journal Nature Communications, the team together with collaborators at Cornell University and the University of Missouri focused on the late phase of retrovirus replication. "It is a long way from an infected cell to the mature virus particle that can infect another cell," explains first author Martin Obr. A new particle buds from the cell in an immature, non-infectious state. It then forms a protective shell, a so-called capsid, around its genetic information and becomes infectious. This protective shell consists of a protein, which is organized in hexamers and a few pentamers. The team discovered that a small molecule called IP6 plays a major role in stabilizing the protein shell within the Rous sarcoma virus. "If the protective shell is not stable, the genetic information of the virus could be released prematurely and will be destroyed, but if it's too stable the genome can't exit at all and, therefore, becomes useless," says Assistant Professor Florian Schur. In a previous study, he and his colleagues were able to show IP6 is important in the assembly of HIV. Now, the team proved it to be as important in other retroviruses showing just how essential the small molecule is in the virus life cycle. "When building a car, you have all these big metal parts, like the hood, the roof and the doors—the screws are connecting everything. In our case, the big parts are the capsid proteins and the IP6 molecules are the screws," says Obr. Unexpected flexibility Further developing cryo-electron tomography, a technique that allows scientists to look at extremely small samples in their natural state, the team was able to see how variable the shapes formed by capsid proteins are. "Now we ask ourselves: Why does the virus change the shape of its capsid? What is it adapting to?" says postdoc Martin Obr. Different capsid shapes within the same type of virus could point to differences in the infectivity of virus particles. "Whatever happens, happens for a reason, but there is no clear answer yet," says Florian Schur. Further developing the technology to get to the bottom of these highly optimized pathogens remains a challenging and fascinating task for the scientists. Published in Nature Commun. (May 28, 2021) https://doi.org/10.1038/s41467-021-23506-0
|

|
Scooped by
Juan Lama
|
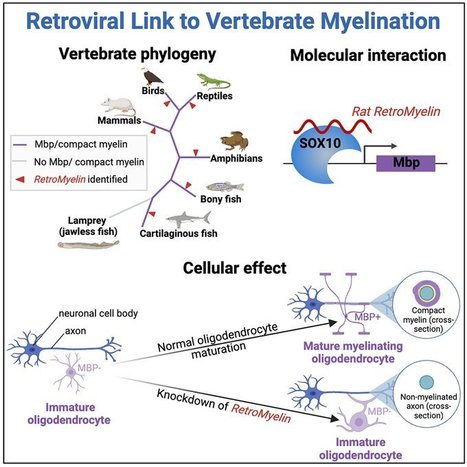
Highlights - RNA expression of retroviral element RNLTR12-int is crucial for myelination
- RNLTR12-int binds to SOX10 to regulate Mbp expression
- RNLTR12-int-like sequences (RetroMyelin) were identified in all jawed vertebrates
- Convergent evolution likely led to RetroMyelin acquisition, adapted for myelination
Summary Myelin, the insulating sheath that surrounds neuronal axons, is produced by oligodendrocytes in the central nervous system (CNS). This evolutionary innovation, which first appears in jawed vertebrates, enabled rapid transmission of nerve impulses, more complex brains, and greater morphological diversity. Here, we report that RNA-level expression of RNLTR12-int, a retrotransposon of retroviral origin, is essential for myelination. We show that RNLTR12-int-encoded RNA binds to the transcription factor SOX10 to regulate transcription of myelin basic protein (Mbp, the major constituent of myelin) in rodents. RNLTR12-int-like sequences (which we name RetroMyelin) are found in all jawed vertebrates, and we further demonstrate their function in regulating myelination in two different vertebrate classes (zebrafish and frogs). Our study therefore suggests that retroviral endogenization played a prominent role in the emergence of vertebrate myelin. Published in Cell (Feb. 15, 2024):

|
Scooped by
Juan Lama
|
Human endogenous retroviruses (HERVs) are ancestral viral relics that constitute nearly 8% of the human genome. Although normally silenced, the most recently integrated provirus HERV-K (HML-2) can be reactivated in certain cancers. Here, we report pathological expression of HML-2 in malignant gliomas in both cerebrospinal fluid and tumor tissue that was associated with a cancer stem cell phenotype and poor outcomes. Using single-cell RNA-Seq, we identified glioblastoma cellular populations with elevated HML-2 transcripts in neural progenitor–like cells (NPC-like) that drive cellular plasticity. Using CRISPR interference, we demonstrate that HML-2 critically maintained glioblastoma stemness and tumorigenesis in both glioblastoma neurospheres and intracranial orthotopic murine models. Additionally, we demonstrate that HML-2 critically regulated embryonic stem cell programs in NPC-derived astroglia and altered their 3D cellular morphology by activating the nuclear transcription factor OCT4, which binds to an HML-2–specific long-terminal repeat (LTR5Hs). Moreover, we discovered that some glioblastoma cells formed immature retroviral virions, and inhibiting HML-2 expression with antiretroviral drugs reduced reverse transcriptase activity in the extracellular compartment, tumor viability, and pluripotency. Our results suggest that HML-2 fundamentally contributes to the glioblastoma stem cell niche. Because persistence of glioblastoma stem cells is considered responsible for treatment resistance and recurrence, HML-2 may serve as a unique therapeutic target. Published in JCI (July 3, 2023): https://doi.org/10.1172/JCI167929

|
Scooped by
Juan Lama
|
Researchers assess the impact of SARS-CoV-2 infection on endogenous retroviruses of the LTR69 subfamily. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which is otherwise known as the coronavirus disease 2019 (COVID-19), continues to pose significant worldwide health and economic effects. There remains an urgent need to better comprehend the intricate relationship between SARS-CoV-2, infected host cells, and the pathophysiology of the disease. Recent research suggests that transposable elements (TEs) play a crucial role in the host's response to COVID-19 and the development of illness. In a recent study posted to the bioRxiv* preprint server, researchers assess the impact of SARS-CoV-2 infection on endogenous retroviruses of the LTR69 subfamily. About the study In the present study, researchers examine the effect of SARS-CoV-2 on the expression profiles of TEs in virus-exposed or -infected cells. To this end, the team investigated publicly available poly(A)-enriched messenger ribonucleic acid (mRNA)-seq data from cell lines and COVID-19 patients to determine the impact of COVID-19 on TE activity. Initially, data collected from SARS-CoV-2-infected and uninfected Calu-3 cells were employed to detect TEs with differential expression. To examine the enhancer activity of long terminal repeat (LTR)-103 and LTR69 in the absence and presence of SARS-CoV-2, the team analyzed publicly available ChIP-seq data associated with Histone H3 Lysine 27 acetylation (H3K27ac) in A549-angiotensin-converting enzyme 2 (ACE2) cells. To determine whether any of the SARS-CoV-2-activated LTR69 repeats elicit regulatory influences, their potential enhancer activities were examined. Five representative candidates were inserted into enhancer reporter vectors. To explore the mechanisms that may be involved in LTR69-Dup69 activation following SARS-CoV-2 infection, the viral nucleotide sequence was examined for binding sites associated with transcription factors known to be active in infected cells. Results Solo-LTRs found in two human endogenous retroviruses (HERV) subfamilies, LTR103_Mam and LTR69, were considerably up-regulated by SARS-CoV-2 infection. Similarly, A549-ACE2 lung cells infected with SARS-CoV-2 and bronchoalveolar lavage fluid (BALF) obtained from non-deceased and deceased SARS-CoV-2 patients exhibited higher LTR69 expression. In contrast, there was no remarkable enhancement in LTR69 expression in SARS-CoV-2-infected Calu-3 cells or in peripheral blood mononuclear cells (PBMCs) of surviving COVID-19 patients. The transcription start site (TSS) profile plot over LTR69 loci revealed enrichment of H3K27Ac marks in infected cells as compared to uninfected cells. No considerable enrichment of enhancer marks was observed on the LTR103_Mam loci. Subsequent investigations were primarily focused on individual LTR69 loci, for which the MACS2 peak calling method detected a minimum of one significant H3K27Ac peak. There were 12 distinct peaks associated with H3K27Ac on 15 LTR69 loci following SARS-CoV-2 infection. LTR12C_GBP2 boosted the expression of Gaussia luciferase relative to the vector control that lacked an LTR repeat. Dup69 had a comparable boosting impact, while the remaining LTR69 elements exhibited no remarkable modulatory impact or even lowered reporter gene expression. Dup69 resides in an intron of protein tyrosine phosphatase receptor type N2 (PTPRN2), which is approximately 500 nucleotides upstream of a long non-coding RNA gene called ENSG00000289418, according to an examination of the respective gene locus. PTPRN2 encodes a tyrosine phosphatase receptor that is a significant autoantigen in type 1 diabetes. In A549-ACE2 and Calu-3 cells, lncRNA expression rose by a factor of 25.2 and 3.6, respectively. In addition, PTPRN2 expression increased by an average of 4.1 times in A549-ACE2 cells following SARS-CoV-2 infection, whereas PTPRN2 mRNA was undetectable in Calu-3 cells. Interestingly, a previous study discovered the up-regulation of PTPRN2 in whole blood samples of COVID-19 patients. Collectively, these results indicate that SARS-CoV-2 infection activates an LTR69 repeat that responded to interferon regulatory transcription factor (IRF)-3 and p65/RelA functions as an enhancer element and may influence the expression of nearby genes. Several probable binding sites for nuclear factor kappa B (NF-κB) subunits, signal transducer and activator of transcription 1 (STAT1), and IRF3 were identified. Furthermore, p65/RelA and an active mutant of IRF3, but not STAT1, may augment the LTR69-mediated increase in reporter gene expression. Consistent with the activation of IRF3 and NF-κB upon innate sensing, synthetic double-stranded RNA analog polyI:C dramatically boosted LTR69_Dup69 activity. Conclusions The study findings showed the differential expression and activation of distinct mobile genetic elements in response to SARS-CoV-2 infection. Specifically, the team identified and validated the infection-induced upregulation of the LTR69 subfamily of endogenous retroviruses. LTR69-Dup69 was also found to possess enhancer activity and exhibit sensitivity to the transcription factors IRF3 and p65/RelA. LTR69 is considered a TE that is activated in SARS-CoV-2-infected cells and can regulate host gene expression, thus contributing to the outcome of COVID-19. However, additional research is required to confirm this finding. Research cited available in bioRxiv (March 21, 2023): https://doi.org/10.1101/2023.03.21.533610

|
Scooped by
Juan Lama
|
Using a next generation sequencing analysis to examine human endogenous retrovirus (HERV) integration sites, researchers from Kumamoto University, the National Institute of Genetics (Japan), and the University of Michigan (U.S.) have discovered that these ancient retroviruses can undergo retrotransposition (DNA sequence insertion with RNA mediation) into iPS cells. The team believes that their discovery places a spotlight on a possible risk that HERVs pose when using iPS cells in regenerative medicine. The study of ancient retroviruses embedded in our genome requires knowledge about our coexistence with viral threats throughout history. We know that HERVs occupy approximately 8% of the human genome and obtain mutations and deletions over long periods. HERVs are also expressed in early embryos and play several physiological roles in human development. For example, HERV-W and HERV-FRD Env proteins are important for placental formation, and HERV-K is thought to protect host cells from exogenous retrovirus infection. However, uncontrollable HERV-K expression is also thought to be associated with various diseases, including various cancers and neurological diseases, but the details of this association is not well known in humans. Since no one has yet discovered replication competent HERVs in our genome, it is thought that they are from an extinct (fossil) virus. In their current work, the research team from Japan and the US discovered that HERV-K is expressed in SOX2-expressing cells, such as those in early embryos, cancer stem cells and iPS cells. They also found that some HERV-K are newly integrated into the host genome in the absence of Env, the viral envelope glycoprotein. This integration was dependent on reverse transcriptase, integrase and protease, thus the researchers hypothesized that the HERV-K embedded in our genome is actually not from a fossil virus, but moves on the genome through the synthesis of proviral DNA reverse transcription. Interestingly, when the researchers compared the HERV-K integration sites between iPS and fibroblast cells from the same donor, they found new HERV-K integration sites in iPS cells. However, the new integration sites were rarely preserved and disappeared during long-term culturing. HERV-K is likely to be randomly integrated into genome, thus the possibility remains that HERV-K retrotransposed-cells predominantly survive depending on their integration site. The movement of HERV-K on the genome might cause cancer and neurological diseases by altering the gene expression profile. The researchers believe that the risk of HERV-K transposition is low in iPS cells but suggest that monitoring HERV-K integration sites should be seriously considered to improve the safety of regenerative medicine using iPS cells. This research was published online on 14 April 2022 in the Journal of Virology. Research cited published in J. Virology (April 14, 2022): https://doi.org/10.1128/jvi.00356-22

|
Scooped by
Juan Lama
|
Many animals, including humans, have DNA left over from ancient viral infections. In koalas, researchers are studying the process in real time. Koalas have been running into hard times. They have suffered for years from habitat destruction, dog attacks, automobile accidents. But that’s only the beginning. They are also plagued by chlamydia and cancers like leukemia and lymphoma, and in researching those problems, scientists have found a natural laboratory in which to study one of the hottest topics in biology: how viruses can insert themselves into an animal’s DNA and sometimes change the course of evolution. The target of this research is Koala retrovirus, or KoRV, a bit of protein and genetic material in the same family as H.I.V. that began inserting itself into the koala genome about 40,000 years ago and is now passed on from generation to generation, like genes. It is also still passed from animal, as a typical viral infection. In recent years, scientists have found that the insertion of viruses into the genomes of animals has occurred over and over again. An estimated 8 percent of the human genome is made up of viruses left over from ancient infections, ancient as in millions of years ago, many of them in primate ancestors before human beings existed. The koala retrovirus is unusual because 40,000 years is the blink of an eye in evolutionary time, and because the process appears to be continuing. A group of scientists reported in Cell on Thursday that they observed a genome immune system fighting to render the virus inactive now that it has established itself in the koala DNA. They also reported that koala retrovirus may have activated other ancient viral DNA. All of this activity stirs the pot of mutation and variation that is the raw material for natural selection. Koala genetics are a gold mine, said William Theurkauf, a professor in molecular medicine at the University of Massachusetts Medical School and one of the authors of the report. “What they are going through is the process of what’s driven the evolution of every animal on the planet.” Past viral infections have led to major evolutionary changes, he said. For example: “A gene that is absolutely essential for the placenta was derived from the shell of a virus millions of years ago.” Humans would not exist without that ancient retroviral infection. Retroviruses are made of RNA, a single strand of genetic information. When they infect a cell, they translate themselves into DNA, the two-stranded molecule that carries all the information for making humans, koalas and other animals. The retroviruses take over the DNA machinery to make more of themselves, which keeps the process going. That process makes us and other animals sick. AIDS is probably the best known retroviral disease. But when the insertion of a retrovirus occurs in a sperm or an egg cell, the change can become permanent, passed on forever. When retroviruses become part of an animal’s inherited DNA, they are called endogenous and eventually they no longer cause the kind of original infection they once did. But they can still be used by the animal's genetic machinery for other purposes, like making a placenta... Additional information published in Cell on October 10, 2019: https://doi.org/10.1016/j.cell.2019.09.002
|



 Your new post is loading...
Your new post is loading...