Your new post is loading...

|
Scooped by
Juan Lama
|
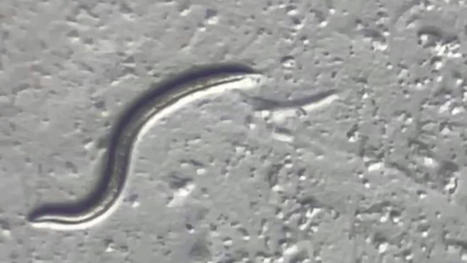
The animal is a previously unknown species that may help researchers unlock secrets of surviving harsh environments. A female microscopic roundworm that spent the last 46,000 years in suspended animation deep in the Siberian permafrost was revived and started having babies in a laboratory dish. By sequencing the genome of this Rip Van Winkle roundworm, scientists revealed it to be a new species of nematode, which is described in a study published Thursday in the journal PLOS Genetics. Nematodes today are among the most ubiquitous organisms on Earth, inhabiting the soil, the water and the ocean floor. “The vast majority of nematode species have not been described,” William Crow, a nematologist at the University of Florida who was not involved in the study, wrote in an email. The ancient Siberian worm could be a species that has since gone extinct, he said. “However, it very well could be a commonly occurring nematode that no one got around to describing yet." Published in Plos Genetics (July 27, 2023): https://doi.org/10.1371/journal.pgen.1010798

|
Scooped by
Juan Lama
|
BUENOS AIRES — President Alberto Fernández of Argentina tested positive for Covid-19 on Saturday and was experiencing mild symptoms despite having been vaccinated earlier this year, becoming the latest in a series of world leaders who have contracted the virus. In a series of tweets sent early Saturday, Mr. Fernández said a “light headache” and a temperature of 99.1 degrees had prompted him to take a quick antigen test. Its positive finding was confirmed later Saturday by a more rigorous P.C.R. test, said Dr. Federico Saavedra, the president’s physician. Mr. Fernández’s symptoms were “mild due in large part to the protective effect of the vaccine,” Dr. Saavedra said. The president, who first learned the preliminary result on Friday, his 62nd birthday, said he would remain in isolation. “I am physically well, and although I would have liked to end my birthday without this news, I’m also in good spirits,” the president wrote on Twitter. Mr. Fernández joins a list of world leaders who have contracted the virus, including Andrés Manuel López Obrador of Mexico, Jair Bolsonaro of Brazil, Emmanuel Macron of France and Donald Trump of the United States, during the final months of his presidency. But Mr. Fernández appears to be the first of those leaders to test positive for Covid-19 after having been fully vaccinated. He received the first dose of Russia’s Sputnik V vaccine on Jan. 21 and the second dose on Feb. 11. Russia’s Gamaleya Institute, which developed Sputnik V, wished the president well and said that while the vaccine has an efficacy rate of 91.6 percent, it is fully effective in preventing critical cases. “The vaccination ensures quick recovery without severe symptoms,” the institute wrote in a statement on Twitter. “We wish you a quick recovery!” Word of Mr. Fernández’s test results comes shortly after Argentina tightened its borders amid an upsurge of Covid-19 infections. Several neighboring countries, particularly Brazil, are experiencing a sharp increase in cases as new, more contagious variants of the virus engulf the region. Argentina recently canceled all direct flights with Brazil, Chile and Mexico in an effort to block the new strains. Argentina was the first country in Latin America to approve the use of the Sputnik V vaccine, in late December, but mass inoculations are taking longer than the government had initially predicted amid a global shortage of the vaccine. The country has also been administering China’s Sinopharm vaccine and Covishield, the Indian version of the AstraZeneca vaccine. Of the nation’s 45 million people, 683,771 have received two vaccine doses, and there have been 4.18 million doses injected over all. Argentina said on March 26 that it would delay applying the second dose of the Covid-19 vaccine for three months in an effort to ensure as many people as possible get at least one dose. The country has reported nearly 2.4 million Covid-19 infections and more than 56,000 deaths.

|
Scooped by
Juan Lama
|
Russia's Sputnik V vaccine is 92% effective at protecting people from COVID-19 according to interim trial results, the country's sovereign wealth fund said on Wednesday, as Moscow rushes to keep pace with Western drugmakers in the race for a shot. Russia's results are only the second from a late-stage human trial, following on swiftly from data released on Monday by Pfizer Inc PFE.N and BioNTech BNTX.O, which said their shot was also more than 90% effective. While experts said the Russian data was encouraging and reinforced the idea the pandemic could be halted by vaccines, they warned that the results were only based on a small number of trial volunteers who had contracted COVID-19. The analysis was conducted after 20 participants developed the virus and examined how many had received the vaccine versus a placebo. That is significantly lower than the 94 infections in the trial of the vaccine being developed by Pfizer and BioNTech. “I assume there was political pressure after the press release from Pfizer and BioNTech earlier in the week to now draw level with their own data,” said Bodo Plachter, deputy director of the Institute of Virology at the Mainz University. “What is missing for now is an analysis of statistical significance.” To confirm the efficacy rate of its vaccine, Pfizer said it would continue its trial until there were 164 COVID-19 cases. The Russian Direct Investment Fund (RDIF), which has been backing Sputnik V’s development, said the Russian trial would continue for six months. Alexander Gintsburg, director of the Gamaleya Institute which developed the vaccine, said the interim results demonstrated that Sputnik V was effective and mass vaccinations would be rolled out in Russia in the coming weeks. In later comments, aired by Rossiya-24 state TV channel, he said at least 1.5 million people in Russia were expected to receive the shot by the end of the year. He added that around 40,000 to 45,000 Russians had already been vaccinated. European stocks and U.S. stock futures extended their gains slightly after Russia’s announcement though the reaction was far more muted than after Pfizer’s results. China’s Sinopharm, which is running large-scale late-stage clinical trials for two COVID-19 vaccine candidates, said on Wednesday that its data was better than expected, though it did not give further details. ‘NOT A COMPETITION’ Successful vaccines are seen as a crucial to restoring daily life around the world by helping end the pandemic that has killed more than 1.26 million people, shuttered businesses and put millions out of work. However, experts said knowledge about the Russian trial’s design was sparse, making it hard to interpret the data. Scientists have raised concerns about the speed at which Moscow has worked, giving the regulatory go-ahead for the shot and launching mass vaccinations before full trials to test its safety and efficacy had been completed. “This is not a competition. We need all trials to be carried out to the highest possible standards and it is particularly important that the pre-set criteria for unblinding the trial data are adhered to avoid cherry picking the data,” said Eleanor Riley, a professor of immunology and infectious disease at the University of Edinburgh.

|
Scooped by
Juan Lama
|
Russia's "Sputnik-V" COVID-19 vaccine produced an antibody response in all participants in early-stage trials, according to results published on Friday by The Lancet medical journal that were hailed by Moscow as an answer to its critics. The results of the two trials, conducted in June-July this year and involving 76 participants, showed 100% of participants developing antibodies to the new coronavirus and no serious side effects, The Lancet said. Russia licensed the two-shot jab for domestic use in August, the first country to do so and before any data had been published or a large-scale trial begun. “The two 42-day trials – including 38 healthy adults each – did not find any serious adverse effects among participants, and confirmed that the vaccine candidates elicit an antibody response,” The Lancet said. “Large, long-term trials including a placebo comparison, and further monitoring are needed to establish the long-term safety and effectiveness of the vaccine for preventing COVID-19 infection,” it said. The vaccine is named Sputnik-V in homage to the world’s first satellite, launched by the Soviet Union. Some Western experts have warned against its use until all internationally approved testing and regulatory steps have been taken. But with the results now published for the first time in an international peer-reviewed journal, and with a 40,000-strong later-stage trial launched last week, a senior Russian official said Moscow had faced down its critics abroad. “With this (publication) we answer all of the questions of the West that were diligently asked over the past three weeks, frankly with the clear goal of tarnishing the Russian vaccine,” said Kirill Dmitriev, the head of the Russian Direct Investment Fund (RDIF), Russia’s sovereign wealth fund, which has backed the vaccine. “All of the boxes are checked,” he told Reuters. “Now... we will start asking questions of some of the Western vaccines.” Commenting on the results of the early-stage trials, lead author Dr Naor Bar-Zeev of the International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, USA said the studies were “encouraging but small”. Bar-Zeev, who was not involved in the study, said “clinical efficacy for any COVID-19 vaccine has not yet been shown.” “The report is a case of ‘so far, so good’,” Brendan Wren, Professor of Microbial Pathogenesis at London’s School of Hygiene and Tropical Medicine said. Original study in The Lancet (Sept. 4, 2020): https://doi.org/10.1016/S0140-6736(20)31866-3

|
Scooped by
Juan Lama
|
Despite Putin’s reported endorsement, little-tested vaccine is not approved for widespread use until 2021. In a startling and confusing move, Russia claimed today it had approved the world’s first COVID-19 vaccine, as the nation’s Ministry of Health issued what’s called a registration certificate for a vaccine candidate that has been tested in just 76 people. The certificate allows the vaccine, developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Moscow, to be given to “a small number of citizens from vulnerable groups,” including medical staff and the elderly, a Ministry of Health spokesperson tells ScienceInsider. But the certificate stipulates that the vaccine cannot be used widely until 1 January 2021, presumably after larger clinical trials have been completed. Scientists around the world immediately denounced the certification as premature and inappropriate, as the Gamaleya vaccine has yet to complete a trial that convincingly shows it is safe and effective in a large group of people. Even some within Russia challenged the move. “It's ridiculous,” says Svetlana Zavidova, a lawyer who heads the Association of Clinical Research Organizations in Russia. “I feel only shame for our country.” Zavidova, who has worked on clinical trials for 20 years and anticipated the approval, yesterday sent an appeal to the Ministry of Health to postpone registering the vaccine until proper efficacy trials are completed. “Accelerated registration will no longer make Russia a leader in this race, it will only expose end users of the vaccine, citizens of the country of the Russian Federation, to unnecessary danger,” she wrote on behalf of the clinical research group. Gamaleya has developed vaccines before, and Mikhail Murashko, the Russian minister of health, said in a government press release that the COVID-19 vaccine showed “high efficacy and safety” and there were no serious side effects. The same release suggested the vaccine would confer 2 years of immunity to SARS-CoV-2, the virus that causes COVID-19. That estimate is apparently based on vaccines Gamaleya has made with similar technology. Russian President Vladimir Putin reportedly endorsed the use of the vaccine, which is dubbed “Sputnik V,” saying it had “passed all necessary steps” and noting that one of his adult daughters had received it. (Putin has not clearly acknowledged his children in public, but he does sometimes refer to them; one is a medical doctor in Moscow.) Putin, who apparently made these comments at a government meeting, added, “I hope we can start a massive release of this vaccine soon.” The Russian registration certificate gives few details about the vaccine, which is being manufactured by Binnopharm in Zelenograd. The company says it can produce 1.5 million doses of the product per year and hopes to expand its manufacturing capacity. More information is available in descriptions of two small trials of the candidate vaccine that appear on ClinicalTrials.gov, a website run by the U.S. National Institutes of Health. The vaccine consists of two shots that use different versions of adenoviruses, some of which cause the common cold, that Gamaleya researchers have engineered to carry the gene for the surface protein, or spike, of SARS-CoV-2. Apparently, the studies are comparing a single shot of adenovirus 26 with the spike gene to a scheme, known as prime-boost, that also gives a second dose 21 days later of a vaccine that contains the spike gene in adenovirus 5. Some vaccine experts have raised concerns about COVID-19 vaccines that use adenovirus 5 in this way. In 2007, researchers stopped an HIV vaccine trial that used adenovirus 5 to shuttle in the gene for the surface protein of that virus after they found that it increased the likelihood of its transmission. In 2017, Gamelaya received approval in Russia for a vaccine that also used the adenovirus 5 vector to deliver the surface protein gene from the virus that causes Ebola. Researchers there used a similar strategy for a vaccine for Middle East respiratory syndrome, a disease caused by a coronavirus like the one responsible for COVID-19. It is still under development and has entered early stage clinical trials. In the United States, the Food and Drug Administration (FDA) can approve the use of medicines prior to the completion of efficacy trials through what’s known as an emergency use authorization, and there has been growing concern that President Donald Trump will push for this with a COVID-19 vaccine to help his re-election prospects in November. Zavidova says Russia’s certification process is similar to FDA’s emergency use. But FDA has an independent advisory committee, largely consisting of academics, that routinely reviews vaccine approval applications.

|
Scooped by
Juan Lama
|
The country’s regulators became the first in the world to approve a possible vaccine against the virus, despite warnings from the global authorities against cutting corners . A Russian health care regulator has become the first in the world to approve a vaccine for the coronavirus, President Vladimir V. Putin announced on Tuesday, though the vaccine has yet to complete clinical trials. The Russian dash for a vaccine has already raised international concerns that Moscow is cutting corners on testing to score political and propaganda points. Mr. Putin’s announcement came despite a caution last week from the World Health Organization that Russia should not stray from the usual methods of testing a vaccine for safety and effectiveness. Mr. Putin’s announcement became essentially a claim of victory in the global race for a vaccine, something Russian officials have been telegraphing for several weeks now despite the absence of published information about any late-phase testing. “It works effectively enough, forms a stable immunity and, I repeat, it has gone through all necessary tests,” Mr. Putin told a cabinet meeting Tuesday morning. He thanked the scientists who developed the vaccine for “this first, very important step for our country, and generally for the whole world.” Western regulators have said repeatedly that they do not expect a vaccine to become widely available before the end of the year at the earliest. Regulatory approval in Russia, well ahead of that timeline, could become a symbol of national pride and provide a much needed political lift for Mr. Putin. The Russian vaccine, along with many others under development in a number of countries in the effort to alleviate a worldwide health crisis that has killed at least 734,900 people, sped through early monkey and human trials with apparent success. Vaccines generally go through three stages of human testing before being approved for widespread use. The first two phases test the vaccine on relatively small groups of people to see if it causes harm and if it stimulates the immune system. The last phase, known as Phase 3, compares the vaccine to a placebo in thousands of people. This final phase is the only way to know with statistical certainty whether a vaccine prevents an infection. And because it’s testing a much larger group of people, a Phase 3 trial can also pick up more subtle side effects of a vaccine that earlier trials could not. The Food and Drug Administration in the United States has said that a new coronavirus vaccine would need to be 50 percent more effective than a placebo in order to be approved. The Russian scientific body that developed the vaccine, the Gamaleya Institute, has yet to conduct Phase III tests on tens of thousands of volunteers in highly controlled trials, a process seen as the only method of ensuring a vaccine is actually safe and effective. Around the world, more than 30 vaccines out of a total of more than 165 under development are now in various stages of human trials. The Russian vaccine uses two strains of adenovirus that typically cause mild colds in humans. They are genetically modified to cause infected cells to make proteins from the spike of the new coronavirus. The approach is similar to a vaccine developed by Oxford University and AstraZeneca that has shown promise in early testing and is now undergoing Phase III tests in Britain, Brazil and South Africa.....
|

|
Scooped by
Juan Lama
|
Agency declines import permit, claiming crippled adenovirus that serves as vaccine is active in second doses. A confusing and unusually nasty fight broke out this week over the safety of a Russian COVID-19 vaccine known as Sputnik V after a Brazilian health agency declined on Monday to authorize its import because of quality and safety concerns. The stakes escalated yesterday when the Twitter account officially associated with the vaccine said “Sputnik V is undertaking a legal defamation proceeding” against Brazil’s regulators. In an online press conference several hours later, the Brazilian Health Regulatory Agency (Anvisa) defended its decision, maintaining that documentation from some of the Russian facilities making Sputnik V shows that one of its two doses contains adenoviruses capable of replication, a potential danger to vaccine recipients. The vaccine uses two different adenoviruses, which cause the common cold, to deliver the gene for the spike protein of SARS-CoV-2, the virus that causes COVD-19. Both are supposed to be stripped of a key gene that allows them to replicate. The Monday announcement left many scientists and media outlets believing Anvisa had directly tested Sputnik V for replicating adenoviruses, which would be unusual for a regulatory agency. But Anvisa has since clarified—it had not and was relying on information provided by the Gamaleya National Center of Epidemiology and Microbiology, the Moscow-based developer of the vaccine. “The data we evaluated shows the presence of replicating virus,” Gustavo Mendes, general manager of medicines and biological products at Anvisa, said at the press conference. Anvisa would not accept the vaccine, he said, without further studies to indicate it is safe. Gamaleya said in a statement on its website that Anvisa’s allegations “have no scientific grounds and cannot be treated seriously.” The research institute added that “no replication-competent adenoviruses (RCA) were ever found in any of the Sputnik V vaccine batches” and said a four-stage purification process prevents contamination. The furor comes as Brazil, which has one of the highest burdens of COVID-19 in the world, is desperately trying to expand its vaccination campaign. The country has vaccinated just 14% of its people with a first dose and governors from some states hoped to bolster that effort by grouping together to buy 30 million doses of Sputnik V. The spat has bewildered and divided outsider observers, in Brazil and elsewhere. Some scientists have used social media to decry the apparent contamination and some have denounced the aggressive response by Sputnik V’s backers, who were already under fire for releasing little data on the vaccine’s safety record. On Wednesday, an agency of the European Union also issued a report criticizing Russia’s promotional effort for Sputnik V for providing disinformation. Other scientists, however, have questioned whether Anvisa appropriately interpreted the information provided by Sputnik V’s makers, and whether the media has too readily accepted the agency’s claim that the vaccine is contaminated. The stakes are high because Sputnik V has been authorized for use in more than 60 countries, although neither the World Health Organization nor the European Medicines Agency has yet authorized it. “We need this vaccine. It’s cheap. It’s effective. It’s easy to store and transport,” says Hildegund Ertl, an adenovirus vaccine scientist at the Wistar Institute. “If the press could just take a deep breath before they rush to conclusions it would really help us all.” One of the scientists who criticized Sputnik V this week on Twitter said she is keeping an open mind. “I will be glad to correct myself in public should the data be shared,” says Angela Rasmussen, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan. (Her Twitter thread about Anvisa’s decision got a response from Sputnik V’s account that read “please do not spread fake news.”) Viruses reborn? The Anvisa review of Sputnik V was triggered because the Brazilian governors needed the agency’s sign-off to import the vaccine. Although complaints about Russia’s lack of transparency with Sputnik V data have simmered for months, many public health officials and scientists worldwide had been reassured when The Lancet recently published results from nearly 20,000 people in a clinical trial. The study showed the vaccine was safe and had an efficacy of 91.6% at preventing symptomatic COVID-19. Both of the adenoviruses that make up Sputnik V, known as Ad5 and Ad26, are churned out by cultured human cells called HEK293 cells. The adenoviruses ferry the coronavirus spike gene to the vaccine recipient’s cells, which then make spike, prompting an immune response. In order to stop the adenoviruses from replicating once inside their human host, the vaccinemaker removed a gene they need for reproduction, called E1. The viruses can copy themselves in HEK293 cells, which are engineered to have a stand-in E1 gene, but they are not supposed to be able to replicate once they are separated from the human cells and packaged in the final vaccine product. It’s long been known that Ad5 can on rare occasions acquire the E1 gene from the HEK293 cells, converting what is supposed to be a crippled virus into an RCA. Although adenoviruses typically cause mild colds, they can rarely kill people, and immunocompromised people who receive a vaccine that inadvertently contains RCAs could be at particular risk. Vaccine makers and others have developed tests to check for replicating adenoviruses in their products. Anvisa said that although the standard worldwide has been zero tolerance for the presence of replicating adenovirus in the vaccine, Gamaleya established an acceptable limit of 5000 replication-capable virus particles per vaccine dose. The Russian quality control documents displayed by Anvisa during the press conference state the batches tested had “less than 100” replication-capable particles per dose. During yesterday’s press conference, Mendes also showed video of parts of an online meeting in March between officials from Anvisa and the vaccine’s developer. In one of the clips, Anvisa officials ask Gamaleya representatives why they had not changed their production methods once they “had detected the RCA occurrence in your production” The Gamaleya representatives responded that they were aware of the risk, but that changing the process “would take too much time.” Mendes noted that Anvisa has analyzed the quality control documentation on other adenovirus-based COVID-19 vaccines, such as those made by AstraZeneca and Johnson & Johnson, and found no evidence of replication-competent viruses in those companies’ final products..... Published in Science (April 30, 2021): https://doi.org/10.1126/science.abj2483

|
Scooped by
Juan Lama
|
A peer-reviewed article in The Lancet shows the vaccine has an impressive 91.6 percent efficacy rate against the virus and is completely protective against severe cases of Covid-19. MOSCOW — Russia cleared a hurdle in its vaccine rollout on Monday with the publication in the respected British medical journal The Lancet of late-stage trial results showing that the country’s Sputnik V vaccine is safe and highly effective. The publication is sure to buoy the Russian government’s promotion of the vaccine at home and around the world, strengthening the Kremlin’s hand in vaccine diplomacy with a credible endorsement of the product’s safety. Russia drew criticism from Western experts when it approved the vaccine for emergency use in August — before late-stage trials had even begun — and started vaccinations that month. Moscow claimed victory in the vaccine race, as it had decades earlier in the space race with the launch of the Sputnik satellite, though at the time other vaccines were further along in testing. In the end, its politicized rollout only served to deepen skepticism. The peer-reviewed article published Tuesday cleared those doubts. It showed the vaccine had an impressive efficacy rate of 91.6 percent against the virus and was completely protective against severe forms of Covid-19. “The development of the Sputnik V vaccine has been criticized for unseemly haste, corner cutting, and an absence of transparency,” two independent researchers, Ian Jones of the University of Reading and Polly Roy with the London School of Hygiene and Tropical Medicine, wrote in a commentary published in The Lancet. “But the outcome reported here,” they continued, “is clear and the scientific principle of vaccination is demonstrated.” Their commentary did note that the design of the Russian vaccine, which relies on a genetically modified cold virus and is similar to half a dozen others including those made by Johnson & Johnson and AstraZeneca, is difficult to mass produce. Though quick out of the gate with regulatory approval, Russia has lagged in mass production and actual vaccinations, the process that in fact protects people from illness and death. While beneficial for speeding global immunity to the disease, the policy has also reaped public relations and diplomatic benefits for the Russian government, even as residents of many provincial Russian cities still do not have access to shots. On Monday, for example, the authorities in the Leningrad region in northwest Russia said supplies had run out. So far, 15 other countries, including Argentina, Hungary and Serbia, have approved the Sputnik V vaccine for emergency use. “Publication in The Lancet today really shows that Sputnik V is the vaccine for all mankind,” Kirill Dmitriev, the director of the Russian Direct Investment Fund, said in a statement. “Today is a great victory.” The vaccine is one of three that have completed late-stage trials showing an efficacy rate above 90 percent, along with the shots made by Pfizer and Moderna. The version of the Russian vaccine tested in the trials must be shipped and stored at difficult-to-manage temperatures below about zero degrees Fahrenheit. The Russian ministry of health has also approved a freeze-dried version that can be stored in a refrigerator. Russia is marketing Sputnik V at a price of about $10 per dose for the two-shot vaccine. The clinical trial conducted in Moscow late last year on about 20,000 volunteers showed only side effects commonly associated with vaccines, such as headaches or mild fevers. The researchers determined that no so-called adverse events, or serious medical problems among the trial participants, were associated with the vaccine. In total, they found 70 serious medical episodes in 68 people in the trial, in both the placebo and vaccine group. Notably, two people who were administered the vaccine died of Covid-19 following illnesses that began days after the first injection. The researchers said both people were likely infected before the trial began and fell ill before the vaccine had time to generate antibodies to the coronavirus. The “disease had progressed before any immunity from the vaccine developed,” they wrote. The Russian authors of The Lancet article also noted the trial in Moscow lacked ethnic diversity to ensure the vaccine is safe in nonwhite recipients. A trial of Sputnik V underway now in the United Arab Emirates includes a more diverse study group, the researchers say. Findings published in The Lancet (Feb. 2, 2021): https://doi.org/10.1016/S0140-6736(21)00234-8

|
Scooped by
Juan Lama
|
The vaccine has only been tested in a small-scale trial with 100 volunteers, results of which are yet to be made available. Russia has granted regulatory approval to a second Covid-19 vaccine—even though the drug has yet to begin large scale Phase-III trials—two months after it greenlit another vaccine that experts expressed caution over due to lack of adequate safety and efficacy data. The second vaccine was announced by Russian President Vladimir Putin at a government meeting on Wednesday, Reuters reported. The peptide-based drug, named EpiVacCorona, has been developed by the Vector Institute in Siberia. A placebo-controlled trial had 100 volunteers, between the ages of 18 and 60, receive the shot in the city of Novosibirsk. Results of those early-stage trials are yet to be published and large-scale Phase-III trials, which help establish safety and efficacy, have not yet begun. Putin said that Russia will increase the production of both the vaccines that have been approved by the government and plans to work with “foreign partners” to promote the vaccine abroad. In August, Russia became the first country to grant regulatory approval to a Covid-19 vaccine, despite limited testing. That vaccine, named-Sputnik-V, was developed by Moscow’s Gamaleya Institute. Scientists expressed concern that without proper trial data, there was no way of knowing if the vaccine was actually effective or safe. The Russian President, however, claimed that the drug was both safe and effective noting that it had already been administered to one of his daughters. Hundreds of people who work in a place that puts them at high risk for getting infected with the coronavirus have been inoculated with the Sputnik-V shot. The vaccine though is not yet in general use. When Sputnik-V was announced, former FDA Commissioner Scott Gotlieb said that Russia was “certainly not” ahead of the U.S. on Covid-19 vaccine development, noting that the U.S. would not allow mass distribution of a drug had been only tested on a few hundred patients at most...

|
Scooped by
Juan Lama
|
High-profile COVID-19 vaccines developed in Russia and China share a potential shortcoming: They are based on a common cold virus that many people have been exposed to, potentially limiting their effectiveness, some experts say. CanSino Biologics’ vaccine, approved for military use in China, is a modified form of adenovirus type 5, or Ad5. The company is in talks to get emergency approval in several countries before completing large-scale trials, the Wall Street Journal reported last week. A vaccine developed by Moscow’s Gamaleya Institute, approved in Russia earlier this month despite limited testing, is based on Ad5 and a second less common adenovirus. “The Ad5 concerns me just because a lot of people have immunity,” said Anna Durbin, a vaccine researcher at Johns Hopkins University. “I’m not sure what their strategy is … maybe it won’t have 70 percent efficacy. It might have 40 percent efficacy, and that’s better than nothing, until something else comes along.” Vaccines are seen as essential to ending the pandemic that has claimed over 845,000 lives worldwide. Gamaleya has said its two-virus approach will address Ad5 immunity issues. Both developers have years of experience and approved Ebola vaccines based on Ad5. Neither CanSino nor Gamaleya responded to requests for comment. Researchers have experimented with Ad5-based vaccines against a variety of infections for decades, but none are widely used. They employ harmless viruses as “vectors” to ferry genes from the target virus — in this case the novel coronavirus — into human cells, prompting an immune response to fight the actual virus. But many people already have antibodies against Ad5, which could cause the immune system to attack the vector instead of responding to the coronavirus, making these vaccines less effective. Several researchers have chosen alternative adenoviruses or delivery mechanisms. Oxford University and AstraZeneca based their COVID-19 vaccine on a chimpanzee adenovirus, avoiding the Ad5 issue. Johnson & Johnson’s candidate uses Ad26, a comparatively rare strain. Dr. Zhou Xing, from Canada’s McMaster University, worked with CanSino on its first Ad5-based vaccine, for tuberculosis, in 2011. His team is developing an inhaled Ad5 COVID-19 vaccine, theorizing it could circumvent pre-existing immunity issues. “The Oxford vaccine candidate has quite an advantage” over the injected CanSino vaccine, he said. Xing also worries that high doses of the Ad5 vector in the CanSino vaccine could induce fever, fueling vaccine skepticism. “I think they will get good immunity in people that don’t have antibodies to the vaccine, but a lot of people do,” said Dr. Hildegund Ertl, director of the Wistar Institute Vaccine Center in Philadelphia. In China and the United States, about 40 percent of people have high levels of antibodies from prior Ad5 exposure. In Africa, it could be has high as 80 percent, experts said. Some scientists also worry an Ad5-based vaccine could increase chances of contracting HIV. In a 2004 trial of a Merck & Co Ad5-based HIV vaccine, people with pre-existing immunity became more, not less, susceptible to the virus that causes AIDS. Researchers, including top U.S. infectious diseases expert Dr. Anthony Fauci, in a 2015 paper, said the side effect was likely unique to HIV vaccines. But they cautioned that HIV incidence should be monitored during and after trials of all Ad5-based vaccines in at-risk populations. Gamaleya’s vaccine will be administered in two doses: The first based on Ad26, similar to J&J’s candidate, and the second on Ad5. Alexander Gintsburg, Gamaleya’s director, has said the two-vector approach addresses the immunity issue. Ertl said it might work well enough in individuals who have been exposed to one of the two adenoviruses.

|
Scooped by
Juan Lama
|
On Tuesday, Russian President Vladimir Putin announced that his country had registered the world's first coronavirus vaccine, named ‘Sputnik V’. The president said that one of his daughters had tested the vaccine on herself. What we know about the vaccine: - Developed by Moscow’s Gamaleya Research Institute of Epidemiology and Microbiology, the dose is based on a platform already used for six other vaccines, according to Russian Minister of Health Mikhail Murashko.
- The Ministry of Health registered ‘Sputnik V’ as a solution to be injected.
- According to the results of clinical studies, the vaccine has shown both high efficacy and safety.
- All the volunteers developed high titers [concentration levels] of antibodies to Covid-19, Murashko noted.
- He also said that “none of them had serious complications” after receiving the vaccine.
- After vaccination, immunity to Covid-19 can last up to two years, the Health Ministry claimed.
How the vaccine will be distributed: - President Putin expects that mass production of the coronavirus vaccine will begin in the near future.
- The solution will be produced at two sites: The Russian Ministry of Health’s Gamaleya Institute and the domestic biopharmaceutical company Binnopharm.
- Some foreign countries have already shown interest in acquiring doses of the Russian vaccine.
Vaccination: - Medical workers and teachers will be the first to receive the vaccine.
- Vaccination of medical professionals could begin in late August or September.
- ‘Sputnik V’ will be available to the general public on January 1, 2021.
- Vaccination will be voluntary in Russia.
The Russian Direct Investment Fund and the Gamaleya Institute of Epidemiology and Microbiology have launched a new website with information about ‘Sputnik V’.

|
Scooped by
Juan Lama
|
A gas explosion has sparked a fire at a Russian bioweapons facility which stores viruses including Ebola Smallpox and Anthrax. The blast occurred on Monday after a gas cylinder exploded during scheduled repair work on the fifth floor of the six-story Russian State Centre for Research on Virology and Biotechnology, commonly known as Vector, the facility said in a statement. No biological material was held in the sanitary inspection room where the explosion occurred, and no structural damage was caused to the concrete laboratory building, the center added. One worker was taken to hospital and is being treated in intensive care for burns, Russia's TASS news agency reported. A fire, covering 30 square meters was later extinguished by fire fighters. The bioweapons facility, located in Koltosvo, in the Novosibirsk region of Siberia, is known for being one of two centers in the world housing samples of smallpox. The US Center for Disease Control and Protection is the only other center in the world known to hold live samples of the disease. Originally, the center was used to develop biological weapons research during the Cold War Soviet era. Now it is one of the largest scientific virological and biotechnological centers in Russia.
|



 Your new post is loading...
Your new post is loading...