The SARS coronavirus 2 (SARS-CoV-2) has caused an ongoing global pandemic with currently 29 million confirmed cases and close to a million deaths. At this time, there are no FDA-approved vaccines or therapeutics for COVID-19, but Emergency Use Authorization has been granted for remdesivir, a broad-spectrum antiviral nucleoside analog. However, remdesivir is only moderately efficacious against SARS-CoV-2 in the clinic, and improved treatment strategies are urgently needed. To accomplish this goal, we devised a strategy to identify compounds that act synergistically with remdesivir in preventing SARS-CoV-2 replication.
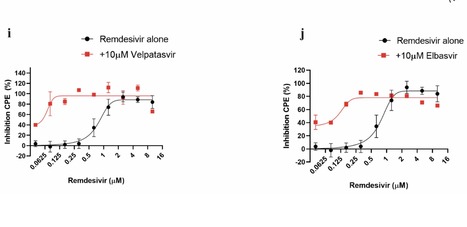
We conducted combinatorial high-throughput screening in the presence of submaximal remdesivir concentrations, using a human lung epithelial cell line infected with a clinical isolate of SARS-CoV-2. We identified 20 approved drugs that act synergistically with remdesivir, many with favorable pharmacokinetic and safety profiles. Strongest effects were observed with established antivirals, Hepatitis C virus nonstructural protein 5 A (HCV NS5A) inhibitors velpatasvir and elbasvir. Combination with their partner drugs sofosbuvir and grazoprevir further increased efficacy, increasing remdesivir's apparent potency 25-fold. We therefore suggest that the FDA-approved Hepatitis C therapeutics Epclusa (velpatasvir/sofosbuvir) and Zepatier (elbasvir/grazoprevir) should be fast-tracked for clinical evaluation in combination with remdesivir to improve treatment of acute SARS-CoV-2 infections.
Preprint available at bioRxiv (Sept. 18, 2020):



 Your new post is loading...
Your new post is loading...