This case series describes rare putative X-chromosomal loss-of-function variants associated with impaired peripheral mononuclear blood cell interferon signaling in 4 young male patients hospitalized with severe coronavirus disease 2019 (COVID-19) in the Netherlands. Severe coronavirus disease 2019 (COVID-19) can occur in younger, predominantly male, patients without preexisting medical conditions. Some individuals may have primary immunodeficiencies that predispose to severe infections caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To explore the presence of genetic variants associated with primary immunodeficiencies among young patients with COVID-19.
Case series of pairs of brothers without medical history meeting the selection criteria of young (age <35 years) brother pairs admitted to the intensive care unit (ICU) due to severe COVID-19. Four men from 2 unrelated families were admitted to the ICUs of 4 hospitals in the Netherlands between March 23 and April 12, 2020. The final date of follow-up was May 16, 2020. Available family members were included for genetic variant segregation analysis and as controls for functional experiments. Results of rapid clinical whole-exome sequencing, performed to identify a potential monogenic cause. Subsequently, basic genetic and immunological tests were performed in primary immune cells isolated from the patients and family members to characterize any immune defects.
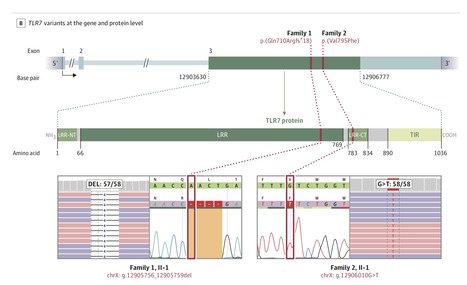
The 4 male patients had a mean age of 26 years (range, 21-32), with no history of major chronic disease. They were previously well before developing respiratory insufficiency due to severe COVID-19, requiring mechanical ventilation in the ICU. The mean duration of ventilatory support was 10 days (range, 9-11); the mean duration of ICU stay was 13 days (range, 10-16). One patient died. Rapid clinical whole-exome sequencing of the patients and segregation in available family members identified loss-of-function variants of the X-chromosomal TLR7. In members of family 1, a maternally inherited 4-nucleotide deletion was identified (c.2129_2132del; p.[Gln710Argfs*18]); the affected members of family 2 carried a missense variant (c.2383G>T; p.[Val795Phe]). In primary peripheral blood mononuclear cells from the patients, downstream type I interferon (IFN) signaling was transcriptionally downregulated, as measured by significantly decreased mRNA expression of IRF7, IFNB1, and ISG15 on stimulation with the TLR7 agonist imiquimod as compared with family members and controls. The production of IFN-γ, a type II IFN, was decreased in patients in response to stimulation with imiquimod. In this case series of 4 young male patients with severe COVID-19, rare putative loss-of-function variants of X-chromosomal TLR7 were identified that were associated with impaired type I and II IFN responses. These preliminary findings provide insights into the pathogenesis of COVID-19.
Published in JAMA (July 24, 2020):



 Your new post is loading...
Your new post is loading...