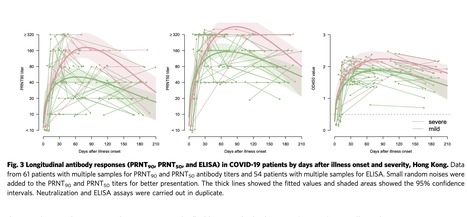
The SARS-CoV-2 pandemic poses the greatest global public health challenge in a century. Neutralizing antibody is a correlate of protection and data on kinetics of virus neutralizing antibody responses are needed. We tested 293 sera from an observational cohort of 195 reverse transcription polymerase chain reaction (RT-PCR) confirmed SARS-CoV-2 infections collected from 0 to 209 days after onset of symptoms. Of 115 sera collected ≥61 days after onset of illness tested using plaque reduction neutralization (PRNT) assays, 99.1% remained seropositive for both 90% (PRNT90) and 50% (PRNT50) neutralization endpoints. We estimate that it takes at least 372, 416 and 133 days for PRNT50 titres to drop to the detection limit of a titre of 1:10 for severe, mild and asymptomatic patients, respectively. At day 90 after onset of symptoms (or initial RT-PCR detection in asymptomatic infections), it took 69, 87 and 31 days for PRNT50 antibody titres to decrease by half (T1/2) in severe, mild and asymptomatic infections, respectively. Patients with severe disease had higher peak PRNT90 and PRNT50 antibody titres than patients with mild or asymptomatic infections. Age did not appear to compromise antibody responses, even after accounting for severity. We conclude that SARS-CoV-2 infection elicits robust neutralizing antibody titres in most individuals.
Published in Nature (Jan. 4, 2021):



 Your new post is loading...
Your new post is loading...