Your new post is loading...

|
Scooped by
Juan Lama
|
More than 4 years into the global COVID-19 pandemic, widespread infection with severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) has left millions of people around the world with Long Covid, which describes the constellation of post-acute and long-term adverse health effects caused by the infection. Evidence generated by the scientific community—with formidable contributions from patient-led research teams—has provided a thorough understanding of the epidemiology and clinical manifestations of Long Covid. Understanding the biologic underpinnings of this disease is also improving, along with evidence that vaccination and antivirals can help prevent it. Yet despite this progress, prevention efforts have stalled, there is uncertainty about governments’ long-term commitment to address research needs in this area, and there has yet to be a treatment option validated with randomized controlled trials (RCTs). Long Covid can manifest in people across the life span (from children to older adults) and across race and ethnicity, sex, and baseline health status. It is a complex nonmonolithic multisystemic disease with sequelae across almost all organ systems. Long Covid is likely a disease with many subtypes that may have different risk factors (genetic, environmental, etc.) and distinct biologic mechanisms that may respond differently to treatments. For example, the prototypical (classic) form of Long Covid (with brain fog, fatigue, dysautonomia, and postexertional malaise) is more common in younger adults and in females. Other forms of Long Covid, including those with cardiovascular and metabolic sequelae, are manifest more often in older adults and those with comorbidities. A common risk across all types of Long Covid is severity of acute infection; the risk—on the relative scale—increases according to the severity of the acute infection. However, despite the lower relative risk, more than 90% of cases occur in people who had mild SARS-CoV-2 infection, owing to the much higher prevalence of mild cases....

|
Scooped by
Juan Lama
|
The virtual study, which included wearable data form more than 30,500 participants, found that changes in sleep, activity and heart rate levels, along with self-reported symptom data, could be used to identify potential cases of Covid-19. Could wearables be used to detect potential Covid-19 cases? A group of researchers at the Scripps Research Translational Institute found that changes in sleep, activity levels and heart rate, paired with symptom data, could be used to identify Covid-19 cases. Their results were published in Nature on Thursday. The idea behind the study was to provide a more effective way to detect potential cases of Covid-19 than the mix of temperature screenings and symptom checklists that many businesses and schools currently use. Temperature alone is not a good indicator — according to a study of hospitalized Covid-19 patients in New York, less than a third of them had an elevated temperature when they were admitted. “We want to do something more than is done now — checking temperature and symptoms. We think that is not enough,” said Giorgio Quer, the study’s first author and director of artificial intelligence at the Scripps Research Translational Institute. “The goal here is really early identification of Covid-positive to slow down the spread.” More than 30,500 people enrolled in the app-based study between late March and early June. They reported symptoms and test results in the app, and consented to sharing anonymized data on their heart rates, sleep and activity levels from their wearable devices. A baseline for each individual’s heart rate, sleep and activity level was calculated for the study. With this data and reported symptoms, a model was able to predict with 80% accuracy whether a person who experienced symptoms was likely to have Covid-19. In particular, researchers found a significant difference in sleep and activity levels for people who tested positive for Covid-19, compared to participants who reported symptoms but tested negative. “A change in your baseline, that’s what’s indicative of something happening,” Quer said. “We saw that for sleep, activity and resting heart rate. It can be a sign of an infection.” The study still had some limitations, including the small number of people who reported a test result. Also, people who own smartwatches or activity trackers might not be reflective of the general population, including groups who have been most affected by the pandemic. A smaller portion of participants in the study reported lower incomes or were older than age 50. Researchers are recruiting more people for the DETECT study, with the goal of enrolling more than 100,000. In particular, they hope to include data from more essential workers, who face an increased risk of exposure to the virus.. Original study published in Nat. Medicine (Oct. 29, 2020): https://doi.org/10.1038/s41591-020-1123-x

|
Scooped by
Juan Lama
|
We may not find out whether the vaccines prevent moderate or severe cases of Covid-19. If you were to approve a coronavirus vaccine, would you approve one that you only knew protected people only from the most mild form of Covid-19, or one that would prevent its serious complications? The answer is obvious. You would want to protect against the worst cases. But that’s not how the companies testing three of the leading coronavirus vaccine candidates, Moderna, Pfizer and AstraZeneca, whose U.S. trial is on hold, are approaching the problem. According to the protocols for their studies, which they released late last week, a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death. To say a vaccine works should mean that most people no longer run the risk of getting seriously sick. That’s not what these trials will determine. The Moderna and AstraZeneca studies will involve about 30,000 participants each; Pfizer’s will have 44,000. Half the participants will receive two doses of vaccines separated by three or four weeks, and the other half will receive saltwater placebo shots. The final determination of efficacy will occur after 150 to 160 participants develop Covid-19. But that is only if the trials are allowed to run long enough. Pfizer will look at the accumulating data four times, Moderna twice and AstraZeneca once to determine if efficacy has been established, potentially leading to an early end to the trials. Knowing how a clinical trial defines its primary endpoint — the measure used to determine a vaccine’s efficacy — is critical to understanding the knowledge it is built to discover. In the Moderna and Pfizer trials, even a mild case of Covid-19 — for instance, a cough plus a positive lab test — would qualify and muddy the results. AstraZeneca is slightly more stringent but would still count mild symptoms like a cough plus fever as a case. Only moderate or severe cases should be counted. There are several reasons this is a problem. First, mild Covid-19 is far more common than severe Covid-19, so most of the efficacy data is likely to pertain to mild disease. But there is no guarantee that reducing the risk of mild Covid-19 will also reduce the risk of moderate or severe Covid-19. The reason is that the vaccine may not work equally well in frail and other at-risk populations. Healthy adults, who could form a majority of trial participants, might be less likely to get mild Covid-19, but adults over 65 — particularly those with significant frailty — might still get sick. This is the case with influenza vaccines, which reduce the risk of mild disease in healthy adults. But there is no solid evidence they reduce the number of deaths, which occur largely among older people. In fact, significant increases in vaccination rates over the past decades have not been associated with reductions in deaths. Second, Moderna and Pfizer acknowledge their vaccines appear to induce side effects that are similar to the symptoms of mild Covid-19. In Pfizer’s early phase trial, more than half of the vaccinated participants experienced headache, muscle pain and chills. If the vaccines ultimately provide no benefit beyond a reduced risk of mild Covid-19, they could end up causing more discomfort than they prevent. Third, even if the studies are allowed to run past their interim analyses, stopping a trial of 30,000 or 44,000 people after just 150 or so Covid-19 cases may make statistical sense, but it defies common sense. Giving a vaccine to hundreds of millions of healthy people based on such limited data requires a real leap of faith. Declaring a winner without adequate evidence would also undermine the studies of other vaccines, as participants in those studies drop out to receive the newly approved vaccine. There may well be insufficient data to address the aged and underrepresented minorities. There will be no data for children, adolescents and pregnant women since they have been excluded. Vaccines must be thoroughly tested in all populations in which they will be used. None of this is to say that these vaccines can’t reduce the risk of serious complications of Covid-19. But unless the trials are allowed to run long enough to address that question, we won’t know the answer. The trials need to focus on the right clinical outcome — whether the vaccines protect against moderate and severe forms of Covid-19 — and be fully completed. It is not too late for the companies to do this, and the Food and Drug Administration, which reviewed the protocols, could still suggest modifications. These are some of the most important clinical trials in history, affecting a vast majority of the planet’s population. It’s hard to imagine how much higher the stakes can be to get this right. Cutting corners should not be an option.
|

|
Scooped by
Juan Lama
|
Long COVID is an often debilitating illness that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms have been identified with impacts on multiple organ systems. At least 65 million individuals worldwide are estimated to have long COVID, with cases increasing daily. Biomedical research has made substantial progress in identifying various pathophysiological changes and risk factors and in characterizing the illness; further, similarities with other viral-onset illnesses such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome have laid the groundwork for research in the field. In this Review, we explore the current literature and highlight key findings, the overlap with other conditions, the variable onset of symptoms, long COVID in children and the impact of vaccinations. Although these key findings are critical to understanding long COVID, current diagnostic and treatment options are insufficient, and clinical trials must be prioritized that address leading hypotheses. Additionally, to strengthen long COVID research, future studies must account for biases and SARS-CoV-2 testing issues, build on viral-onset research, be inclusive of marginalized populations and meaningfully engage patients throughout the research process. Long COVID is an often debilitating illness of severe symptoms that can develop during or following COVID-19. In this Review, Davis, McCorkell, Vogel and Topol explore our knowledge of long COVID and highlight key findings, including potential mechanisms, the overlap with other conditions and potential treatments. They also discuss challenges and recommendations for long COVID research and care. Published in Nat Rev Microbiol (Jan. 2023): https://doi.org/10.1038/s41579-022-00846-2

|
Scooped by
Juan Lama
|
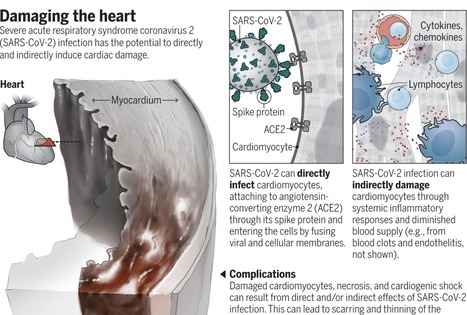
The family of seven known human coronaviruses are known for their impact on the respiratory tract, not the heart. However, the most recent coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has marked tropism for the heart and can lead to myocarditis (inflammation of the heart), necrosis of its cells, mimicking of a heart attack, arrhythmias, and acute or protracted heart failure (muscle dysfunction). These complications, which at times are the only features of coronavirus disease 2019 (COVID-19) clinical presentation, have occurred even in cases with mild symptoms and in people who did not experience any symptoms. Recent findings of heart involvement in young athletes, including sudden death, have raised concerns about the current limits of our knowledge and potentially high risk and occult prevalence of COVID-19 heart manifestations. The four “common cold” human coronaviruses—HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1—have not been associated with heart abnormalities. There were isolated reports of patients with Middle East respiratory syndrome (MERS; caused by MERS-CoV) with myocarditis and a limited number of case series of cardiac disease in patients with SARS (caused by SARS-CoV) (1). Therefore, a distinct feature of SARS-CoV-2 is its more extensive cardiac involvement, which may also be a consequence of the pandemic and the exposure of tens of millions of people to the virus. What appears to structurally differentiate SARS-CoV-2 from SARS is a furin polybasic site that, when cleaved, broadens the types of cells (tropism) that the virus can infect (2). The virus targets the angiotensin-converting enzyme 2 (ACE2) receptor throughout the body, facilitating cell entry by way of its spike protein, along with the cooperation of the cellular serine protease transmembrane protease serine 2 (TMPRSS2), heparan sulfate, and other proteases (3). The heart is one of the many organs with high expression of ACE2. Moreover, the affinity of SARS-CoV-2 to ACE2 is significantly greater than that of SARS (4). The tropism to other organs beyond the lungs has been studied from autopsy specimens: SARS-CoV-2 genomic RNA was highest in the lungs, but the heart, kidney, and liver also showed substantial amounts, and copies of the virus were detected in the heart from 16 of 22 patients who died (5). In an autopsy series of 39 patients dying from COVID-19, the virus was not detectable in the myocardium in 38% of patients, whereas 31% had a high viral load above 1000 copies in the heart (6). Accordingly, SARS-CoV-2 infection can damage the heart both directly and indirectly (see the figure). SARS-CoV-2 exhibited a striking ability to infect cardiomyocytes derived from induced pluripotent stem cells (iPSCs) in vitro, leading to a distinctive pattern of heart muscle cell fragmentation, with “complete dissolution of the contractile machinery” (7). Some of these findings were verified from patient autopsy specimens. In another iPSC study, SARS-CoV-2 infection led to apoptosis and cessation of beating within 72 hours of exposure (8). Besides directly infecting heart muscle cells, viral entry has been documented in the endothelial cells that line the blood vessels to the heart and multiple vascular beds. A secondary immune response to the infected heart and endothelial cells (endothelitis) is just one dimension of many potential indirect effects. These include dysregulation of the renin-angiotensin-aldosterone system that modulates blood pressure, and activation of a proinflammatory response involving platelets, neutrophils, macrophages, and lymphocytes, with release of cytokines and a prothrombotic state. A propensity for clotting, both in the microvasculature and large vessels, has been reported in multiple autopsy series and in young COVID-19 patients with strokes... Published in Science (Sept. 23, 2020): https://doi.org/10.1126/science.abe2813
|




 Your new post is loading...
Your new post is loading...