Your new post is loading...

|
Scooped by
Juan Lama
|
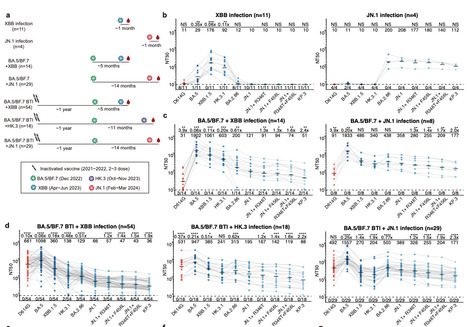
The ongoing evolution of SARS-CoV-2 continues to challenge the global immune barrier established by infections and vaccine boosters. Recently, the emergence and dominance of the JN.1 lineage over XBB variants have prompted a reevaluation of current vaccine strategies. Despite the demonstrated effectiveness of XBB-based vaccines against JN.1, concerns persist regarding the durability of neutralizing antibody (NAb) responses against evolving JN.1 subvariants. In this study, we compared the humoral immunogenicity of XBB and JN.1 lineage infections in human subjects with diverse immune histories to understand the antigenic and immunogenic distinctions between these variants. Similar to observations in naive mice, priming with XBB and JN.1 in humans without prior SARS-CoV-2 exposure results in distinct NAb responses, exhibiting minimal cross-reactivity. Importantly, breakthrough infections (BTI) with the JN.1 lineage induce 5.9-fold higher neutralization titers against JN.1 compared to those induced by XBB BTI. We also observed notable immune evasion of recently emerged JN.1 sublineages, including JN.1+R346T+F456L, with KP.3 showing the most pronounced decrease in neutralization titers by both XBB and JN.1 BTI sera. These results underscore the challenge posed by the continuously evolving SARS-CoV-2 JN.1 and support the consideration of switching the focus of future SARS-CoV-2 vaccine updates to the JN.1 lineage. Preprint in bioRxiv (April 22, 2024): https://doi.org/10.1101/2024.04.19.590276

|
Scooped by
Juan Lama
|
The SARS-CoV-2 variant JN.1 swiftly became the global dominant strain due to a spike protein Leu455Ser substitution, boosting transmissibility and immune-escape capabilities, surpassing its predecessor BA.2.86 and other variants. These alterations have resulted in a surge of COVID-19 cases, reflected in wastewater-surveillance data surpassing rates, observed during the initial omicron wave. However, concerns persist that JN.1 might have an increased capacity to replicate in the gut, potentially leading to infected individuals shedding a higher number of viral copies than previously seen. As there is currently a lack of available data for fecal viral shedding, we are presenting the initial longitudinal and quantitative faecal shedding data for SARS-CoV-2 RNA in individuals infected with XBB.1.5, EG.5.1, HV.1, JD.1.1, BA.2.86, and JN.1. 856 faecal samples were obtained from 113 non-hospitalised individuals with confirmed PCR positivity for SARS-CoV-2 RNA. Variants were identified through Sanger sequencing. Detailed protocols for processing and extracting SARS-CoV-2 RNA from stool samples are provided in the appendix... Published in The Lancet Infectious Diseases (March 21, 2024):

|
Scooped by
Juan Lama
|
In November 2023, SARS-CoV-2 XBB descendants, including EG.5.1 (XBB.1.9.2.5.1), the currently predominant lineage, are circulating worldwide according to Nextstrain. EG.5.1 has a characteristic amino acid substitution in the spike protein (S), S:F456L, which contributes to its escape from humoral immunity. EG.5.1 has further evolved, and its descendant lineage harboring S:L455F (i.e., EG.5.1+S:L455F) emerged and was named HK.3 (XBB.1.9.2.5.1.1.3). HK.3 was initially discovered in East Asia and is rapidly spreading worldwide. Notably, the XBB subvariants bearing both S:L455F and S:F456L substitutions, including HK.3, are called the FLip variants. These FLip variants, such as JG.3 (XBB.1.9.2.5.1.3.3), JF.1 (XBB.1.16.6.1) and GK.3 (XBB.1.5.70.3), have emerged convergently, suggesting that the acquisition of these two substitutions confers a growth advantage to XBB in the human population. Here, we investigated the virological properties of HK.3 as a representative of the FLip variants. Preprint in bioRxiv (Nov. 15, 2023): https://doi.org/10.1101/2023.11.14.566985

|
Scooped by
Juan Lama
|
BA.2.86, nicknamed Pirola, causing concern among scientists because of fear it could be more transmissible. The latest Covid-19 variant, BA.2.86, appears to be spreading in the UK, health surveillance data suggests. The variant, nicknamed Pirola, has prompted concern among scientists because of the high number of mutations it carries, which raises the possibility that it could evade the immune system more easily or be more transmissible. In a briefing note on Friday, the UK Health Security Agency (UKHSA) said that an outbreak at a care home in Norfolk and other cases across the UK indicated there was likely to be community transmission of the strain, but said it was too early to judge the full extent of its spread. In an outbreak of Covid-19 in a care home in Norfolk at the end of August, 33 out of 38 residents tested positive for the virus, along with 12 members of staff, the UKHSA said. One resident needed hospital treatment but no deaths were reported. Laboratory tests later showed that 22 residents had the BA.2.86 variant, along with six staff. The outbreak was “an early indicator” that the variant may be sufficiently transmissible to have an effect in close-contact settings, the UKHSA said, though it was too early to draw any conclusions about how BA.2.86 would behave in the wider UK population. Twenty-nine of the 33 residents at the care home who tested positive for Covid-19 have recovered, along with all members of staff, the UKHSA added. Dr Renu Bindra, the UKHSA incident director, said that while BA.2.86 had a “significant number of mutations” compared with other variants circulating, the data so far was “too limited to draw firm conclusions” about the impact this would have on the transmissibility or severity of the virus. “It is clear that there is some degree of widespread community transmission, both in the UK and globally, and we are working to ascertain the full extent of this,” she said. “In the meantime, it remains vital that all those eligible come forward to receive their autumn vaccine as soon as it is offered to them.” Some early lab-based evidence has eased initial concerns about the potential of BA.2.86 to cause a new global wave of infection, as happened with the emergence of Omicron. A pre-print study, from researchers in China, found that BA.2.86 is not as efficient at infecting cells in the lab compared with other circulating Omicron subvariants. Another pre-print study from researchers in Sweden found only modest drops in how well serum from blood donors could neutralise BA.2.86 compared with other variants. The latest Covid-19 vaccine booster programme has been brought forward from October to September as a precaution against BA.2.86. The booster programme will begin in England on 11 September, with jabs offered first to residents of adult care homes and clinically vulnerable people, before it is extended to everyone in the UK aged 65 and above. See also latest UK SARS-CoV-2 Surveillance Report (Sept. 4, 2023): https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings/sars-cov-2-variant-surveillance-and-assessment-technical-briefing-53

|
Scooped by
Juan Lama
|
EG.5 is spreading quickly, but experts say it’s no more dangerous than previous versions. Concern is rising about the Covid-19 variant EG.5. This week, it became the dominant variant in the United States, and the World Health Organization classified EG.5 as a “variant of interest,” meaning it has genetic changes that give it an advantage and its prevalence is growing. So how worried should people be about it? While severe illness in older adults and people with underlying conditions is always a concern, as is long Covid in anyone who gets infected, experts say EG.5 does not pose a substantial threat — or at least no more of one than any of the other major variants currently circulating. “It’s a concern that it’s increasing, but it doesn’t look like something that’s vastly different from what’s already been circulating in the U.S. for the past three to four months,” said Andrew Pekosz, a professor of molecular microbiology and immunology at Johns Hopkins University Bloomberg School of Public Health. “So I think that’s what tempers my concern about this variant, at this point in time.” Even the W.H.O. stated in its announcement that, based on the available evidence, “the public health risk posed by EG.5 is evaluated as low at the global level.”....

|
Scooped by
Juan Lama
|
As of July 2023, EG.5.1 (a.k.a. XBB.1.9.2.5.1), a XBB subvariant bearing the S:Q52H and S:F456L substitutions, alongside the S:F486P substitution (Figure S1A), has rapidly spread in some countries. On July 19, 2023, the WHO classified EG.5 as a variant under monitoring. First, we showed that EG.5.1 exhibits a higher effective reproduction number compared with XBB.1.5, XBB.1.16, and its parental lineage (XBB.1.9.2), suggesting that EG.5.1 will spread globally and outcompete these XBB subvariants in the near future. We then addressed whether EG.5.1 evades from the antiviral effect of the humoral immunity induced by breakthrough infection (BTI) of XBB subvariants and performed a neutralization assay using XBB BTI sera. However, the 50% neutralization titer (NT50) of XBB BTI sera against EG.5.1 was comparable to those against XBB.1.5/1.9.2 and XBB.1.16. Moreover, the sensitivity of EG.5.1 to convalescent sera of XBB.1- and XBB.1.5-infected hamsters was similar to those of XBB.1.5/1.9 and XBB.1.16. These results suggest that the increased Re of EG.5.1 is attributed to neither increased infectivity nor immune evasion from XBB BTI, and the emergence and spread of EG.5 is driven by the other pressures. We previously demonstrated that Omicron BTI cannot efficiently induce antiviral humoral immunity against the variant infected. In fact, the NT50s of the BTI sera of Omicron BA.1, BA.2, and BA.5 against the variant infected were 3.0-, 2.2-, and 3.4-fold lower than that against the ancestral B.1.1 variant, respectively. However, strikingly, we found that the NT50 of the BTI sera of XBB1.5/1.9 and XBB.1.16 against the variant infected were 8.7- and 8.3-fold lower than that against the B.1.1 variant. These results suggest that XBB BTI cannot efficiently induce antiviral humoral immunity against XBB subvariants. Preprint (August 8, 2023) bioRxiv: https://doi.org/10.1101/2023.08.08.552415

|
Scooped by
Juan Lama
|
The World Health Organization (WHO) yesterday added EG.5 to the list of Omicron variants under monitoring (VUM), as most indicators for tracking COVID-19 activity declined, the group said in its latest weekly update. EG.5 is a descendant of XBB.1.9.2, with one extra spike mutation. Global prevalence has been rising since the end of May. The WHO now has seven VUMs. The number of variants of interest remains at two, including XBB.1.5, which is steadily declining, and XBB.1.16, which is holding steady at 20.7% of sequences. US among countries seeing EG.5 activity The United States is one of the countries seeing rising EG.5 proportions. The Centers for Disease Control and Prevention (CDC) said in its last estimates on Jul 8 that EG.5 made up 13% of samples. The WHO said so far there's no evidence that EG.5 is fueling any rises in cases or deaths or that infections involving the virus are more severe. Other than XBB.1.9.2 descendant lineages, no other VUMs are showing rising proportions, the WHO said. Few red flags with hospitalization, death indicators In its illness tracking, the WHO said COVID-19 cases and deaths continue to decline globally, though it added that reduced testing and reporting mean that case trends don't accurately reflect COVID-19 activity, which it said still poses a burden in some countries. Hospitalizations and deaths are more accurate indicators, the WHO said. Of the limited number of countries that regularly report hospitalization data, only one—Malta—had an increase of 20% or more over the past 28 days. Regarding intensive care unit (ICU) admissions for COVID-19, no countries that routinely report data showed an increase of 20% or more over the last month. Only one region reported a rise in deaths over the last 28 days, the Western Pacific. Most of the rise appears to be from an increase in Australia, which reported a small rise in activity during the early months of the Southern Hemisphere winter. At a WHO briefing yesterday on a host of different health issues, Director-General Tedros Adhanom Ghebreyesus, PhD, said though people are better protected by vaccines and prior infection, countries shouldn't let down their guard. "WHO continues to advise people at high risk to wear a mask in crowded places, to get boosters when recommended, and to ensure adequate ventilation indoors," he said. "And we urge governments to maintain and not dismantle the systems they built for COVID-19."

|
Scooped by
Juan Lama
|
Rapid Antigen Tests (RAT) have become an invaluable tool for combating the COVID-19 pandemic. However, concerns have been raised regarding the ability of existing RATs to effectively detect emerging SARS-CoV-2 variants. We compared the performance of eight commercially available, emergency use authorized RATs against the Delta and Omicron SARS-CoV-2 variants using individual patient and serially diluted pooled clinical samples. The RATs exhibited lower sensitivity for Omicron samples when using PCR Cycle threshold (CT) value (a proxy for RNA concentration) as the comparator. Interestingly, however, they exhibited similar sensitivity for Omicron and Delta samples when using quantitative antigen concentration as the comparator. We further found that the Omicron samples had lower ratios of antigen to RNA, which offers a potential explanation for the apparent lower sensitivity of RATs for that variant when using CT value as a reference. Our findings underscore the complexity in assessing RAT performance against emerging variants and highlight the need for ongoing evaluation in the face of changing population immunity and virus evolution. Preprint available in medRxiv (Feb. 10, 2023): https://doi.org/10.1101/2023.02.09.23285583

|
Scooped by
Juan Lama
|
Top SARS-CoV-2 variants with increased resistance to vaccine and therapeutic antibodies. Seven new lineages including BA.2.75, BA.2.75.2, BA.4.6, BF.7, BQ.1, BQ.1.1 and XBB.1 are circulating globally, with different sub-variants growing faster in different regions. Global and US frequencies of the eight variants as of October 29, 2022 are shown. In the US, the combined pool of new variants already represents 50% of the viral genomes sequenced last week. Top graph shows the spike mutations for each lineages appearing in at least 50% of the viral genomes sequenced for each variant. Key mutations in spike conferring escape from antibodies (vaccine or therapeutics) include R346T, K444T, L452R, N460K, and F486S/V. Data from GISAID, US CDC, cov-spectrum.org, and outbreak.info used to generate the charts at https://lnkd.in/g7YYq4y

|
Scooped by
Juan Lama
|
Significance Tracking the animal reservoir of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants is important for understanding the current COVID-19 pandemic and preventing future pandemics. Speculations about the source of the omicron variant are abundant, yet experimental evidence has been scarce. Here, we provide the structural information on how omicron recognizes its mouse receptor. Our study demonstrates that the omicron mutations in the receptor-binding region are structurally adapted to mouse angiotensin-converting enzyme 2 (ACE2), informing an understanding of the origin of the omicron variant and the evolution of SARS-CoV-2. It may facilitate epidemiological surveillance of SARS-CoV-2 in animals to prevent future coronavirus pandemics. Abstract The sudden emergence and rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variant has raised questions about its animal reservoir. Here, we investigated receptor recognition of the omicron’s receptor-binding domain (RBD), focusing on four of its mutations (Q493R, Q498R, N501Y, and Y505H) surrounding two mutational hotspots. These mutations have variable effects on the RBD’s affinity for human angiotensin-converting enzyme 2 (ACE2), but they all enhance the RBD’s affinity for mouse ACE2. We further determined the crystal structure of omicron RBD complexed with mouse ACE2. The structure showed that all four mutations are viral adaptations to mouse ACE2: three of them (Q493R, Q498R, and Y505H) are uniquely adapted to mouse ACE2, whereas the other one (N501Y) is adapted to both human ACE2 and mouse ACE2. These data reveal that the omicron RBD was well adapted to mouse ACE2 before omicron started to infect humans, providing insight into the potential evolutionary origin of the omicron variant. Published in PNAS (Oct.18, 2022):

|
Scooped by
Juan Lama
|
The United States is in a (relative) Covid-19 lull, with cases and hospitalizations falling as the wave driven by the BA.5 lineage of the Omicron variant recedes. But as if we needed a portent of an anticipated fall and winter wave, Covid is on the rise in some European countries. What’s different, at least for now, is that there’s not one variant pushing the wave. Rather, scientists are tracking a bevy of new forms of Omicron, which are jockeying with each other as they compete to become the next dominant strain. Scientists are monitoring more than 300 sublineages of Omicron, World Health Organization officials said this week. To get a sense of what’s happening right now with the evolution of the SARS-CoV-2 virus, STAT spoke with Tom Peacock, a virologist at Imperial College London. The strains virologists are tracking — from BA.2.75.2 to BQ.1.1 to XBB and beyond (“The names are getting ridiculous,” Peacock said) — are themselves descendants of earlier forms of Omicron, such as BA.2 and BA.5. It’s an example of how, since Omicron emerged nearly a year ago, the coronavirus’s evolution has been more akin to the “drift” seen with influenza, rather than the earlier succession of very different variants, from Alpha to Delta to the original Omicron. “It’s a little bit like what we would expect to see over a couple years of flu, but crammed into about three months with SARS-CoV-2,” Peacock said. But even as the Omicron lineages continue to splinter, scientists have found the different sublineages are picking up some of the same mutations — what’s called “convergent evolution.” That pattern suggests that those mutations confer an evolutionary advantage, one that would allow the virus to continue to spread among people who have different layers of protection, from vaccination and infections from earlier Omicron lineages. Just how big and damaging of a wave the emerging subvariants will drive can’t be predicted. In the U.S., whatever wave comes will build on a baseline of, as of now, some 390 people dying on average a day. It’s also not clear if one variant will outcompete its cousins, or if different combinations will get footholds in different parts of the world. (We’ll note here that the new subvariants don’t seem to completely reset the pandemic: The immunity people have built up from vaccinations and infections will likely continue to offer strong protection against severe outcomes for most, particularly if they’ve stayed up-to-date with boosters.) But the concern about these newest sublineages is not just that they could drive up cases once more. Already, some monoclonal antibody treatments were rendered useless and had to be abandoned as the virus evolved. And in some lab experiments, the remaining antibody therapies — bebtelovimab, as well as Evusheld — can’t stand up to some of the new variants. (Just on Monday, the Food and Drug Administration warned that Evusheld, which is given to immunocompromised people to bolster their protection as a pre-exposure therapy, can’t neutralize certain SARS-2 variants.) That could leave people at high risk for severe Covid even more vulnerable. Peacock added one note: While it’s possible that the future SARS-2 strains we’ll be dealing with will continue to be descended from Omicron, another Omicron-like event could occur. That is, a variant from a distant part of SARS-2’s family tree could appear suddenly and outcompete everything else in the landscape, just as the original Omicron did last year around Thanksgiving. “We’re also coming up to the one-year anniversary of Omicron, so something else could come and just make everything else extinct,” Peacock said. “We should never forget that SARS-CoV-2 has done that once, and can absolutely do it again. Everyone’s looking at these minute changes in all these sublineages and suddenly Pi comes through and torpedoes the whole lot,” Peacock said, referring to the next letter in the Greek alphabet, which would presumably be given to whatever major variant appears next. Below are excerpts from STAT’s conversation with Peacock, lightly edited for clarity. Generally, what’s happening with the evolution of SARS-2 right now? We’re seeing a fairly unprecedented amount of — not for other viruses, but for SARS-CoV-2 — convergent evolution. In other words, although stuff started off in different places — some BA.2, some BA.5 — everything’s going back in the same direction. They’re getting the same mutations, which implies there’s a very strong selective pressure in the environment right now, which of course is people’s immunity, or that’s what everyone is assuming it is. One thing people might have heard when new variants emerge is that they’re the “most immune evasive yet.” Wouldn’t by definition any variant that emerges and spreads well in our current immune landscape have to be able to evade all that immunity to circulate? Isn’t this what should we expect? Yeah, it is absolutely expected. It’s how drift happens. If it’s expected, is it reason for concern? On top of the impact on therapies, is there any sense yet of what kind of impact these sublineages might have on vaccine effectiveness? Countries in Europe and the U.S. are rolling out these bivalent boosters, which account for Omicron, but earlier forms of Omicron. It’s very hard to say because we don’t really know what the vaccine efficacy is going to be against a completely matched virus. That data takes a while. But it’ll probably be similar to how it’s always been, in that there will be some level of drop of protection against infection and symptomatic disease, because you’ve got a mismatch now. But things like severe disease and death will hold up much better, and there will be a much less dramatic drop and or maybe even not much of a drop at all. Is it surprising that for all the evolution this virus has undergone, it’s still finding room to pick up new mutations and still be able to infect, and replicate, and things like that? I remember earlier in the pandemic hearing experts talk about how there were only so many mutations a virus could tolerate before losing some function. Are we just not there yet? People have gone back and looked at some of the seasonal coronaviruses and you do see that they have a lot of tolerance for mutations, and SARS-CoV-2 is showing to have a lot of tolerance as well, clearly. At least in parts of Europe right now you’re starting to see some resurgence of cases, but it’s not as if there’s some brand new variant driving it. So what’s happening now with transmission, and what might happen as these new variants snowball and BA.5 keeps receding? People are saying that maybe some of the variants that are less good at spreading than some of the new sublineages but that still have some antigenic mutations — so BA.4.6, BF.7, for example, which are quite high in a few European countries — they may be the vanguard of this variant wave that we’re going to get over the winter, but that the really nasty ones are still at, what, a percentage point or a couple percentage points in prevalence. So you have the first ones that will get replaced as the wave comes through by these nastier ones that are currently at lower prevalence. We could end up with a mix, and different countries end up with different mixes. This is on top of schools going back across Europe, a sudden cold wave — so maybe there’s some seasonality — and waning immunity. It’s just everything at once, and it’s unclear what each contribution is. But if variants aren’t driving it now, they will be in a few weeks time, we think.

|
Scooped by
Juan Lama
|
Emerging variants and waning immunity are likely to push infections higher in the northern hemisphere as influenza also makes a comeback. Evidence is building that the northern hemisphere is on course for a surge of COVID-19 cases this autumn and winter. New immune-evading strains of the SARS-CoV-2 Omicron variant, behaviour changes, and waning immunity mean that many countries could soon see large numbers of COVID infections — and potentially hospitalizations — say scientists. Nature explores the factors that might drive a COVID-19 wave — and what countries can do to blunt the effects with the new generation of vaccines that target Omicron. Will there be a COVID-19 wave this autumn and winter? In mid-August, an effort called the COVID-19 Scenario Modelling Hub laid out several possible US scenarios for the coming months. With surges caused by the BA.5 Omicron variant in the rear-view mirror — resulting in high population immunity — the United States could be in for a relatively quiet COVID-19 season, the models suggested, so long as vaccine booster campaigns began quickly and new variants didn’t emerge. Even with a new variant, a big surge in cases wasn’t certain. More than a month on, hospitalizations are declining in line with projections, says Justin Lessler, an infectious-disease epidemiologist at the University of North Carolina at Chapel Hill who leads the modelling effort. But other factors on the horizon could spell trouble. The rollout of new, ‘bivalent’ boosters “has been a little bit slow,” says Lessler. And there are now subtle signs that Omicron is evolving and spawning a new cast of immunity-dodging variants. “It could lead to some upswings as we go into the fall and winter months,” Lessler adds. Some US states are already beginning to see an uptick in cases, notes epidemiologist Jennifer Nuzzo at Brown University in Providence, Rhode Island. The United Kingdom’s weekly population survey of SARS-CoV-2 infections, a gold-standard in COVID data, has also documented an increase in COVID prevalence in its past two reports. Hospitalizations of people who test positive for SARS-CoV-2 are rising quickly — although from low levels — in Britain and other European countries. In the backdrop, a slew of immunity-dodging variants are emerging globally, and researchers think these variants will fuel an autumn–winter wave. Are new variants behind rising case numbers? Probably not yet, says Tom Wenseleers, an evolutionary biologist at the Catholic University of Leuven in Belgium. The current rise in SARS-CoV-2 infections is probably largely because of people’s waning immunity — which offers short-lived protection from infection — as well as increased mixing between people. In many countries including the United Kingdom, social dynamics are nearly back to pre-pandemic levels, say health officials. Factors that cause other respiratory viruses to thrive in cooler months — including extra time spent indoors — could also be at play....

|
Scooped by
Juan Lama
|
Highlights - Booster immunization elicits Omicron sublineage neutralizing activity
- Omicron sublineages demonstrate distinct antibody escape profiles
- Most clinical antibodies are inactive against omicron sublineages
- Identification of broad and potent SARS-CoV-2 antibodies with pan-Omicron activity
Summary SARS-CoV-2 neutralizing antibodies play a critical role in COVID-19 prevention and treatment but are challenged by viral evolution and the emergence of novel escape variants. Importantly, the recently identified Omicron sublineages BA.2.12.1 and BA.4/5 are rapidly becoming predominant in various countries. By determining polyclonal serum activity of 50 convalescent or vaccinated individuals against BA.1, BA.1.1, BA.2, BA.2.12.1, and BA.4/5, we reveal a further reduction of BA.4/5 susceptibility to vaccinee sera. Most notably, delineation of sensitivity to an extended 163-antibody panel demonstrates pronounced antigenic differences with distinct escape patterns among Omicron sublineages. Antigenic distance and/or higher resistance may therefore favor immune escape-mediated BA.4/5 expansion after the first Omicron wave. Finally, while most clinical-stage monoclonal antibodies are inactive against Omicron sublineages, we identify promising antibodies with high pan-SARS-CoV-2 neutralizing potency. Our study provides a detailed understanding of Omicron sublineage antibody escape that can inform on effective strategies against COVID-19. Published in Cell Host Microbe (July 6, 2022):
|

|
Scooped by
Juan Lama
|
The COVID-19 pandemic has affected hundreds of millions of individuals and caused more than six million deaths. The prolonged pandemic duration and the continual inter-individual transmissibility have contributed to the emergence of a wide variety of SARS-CoV-2 variants. Genomic surveillance and phylogenetic studies have shown that substantial mutations in crucial supersites of spike glycoprotein modulate the binding affinity of the evolved SARS-COV-2 lineages to ACE2 receptors and modify the binding of spike protein with neutralizing antibodies. The immunological spike mutations have been associated with differential transmissibility, infectivity, and therapeutic efficacy of the vaccines and the immunological therapies among the new variants. This review highlights the diverse genetic mutations assimilated in various SARS-CoV-2 variants. The implications of the acquired mutations related to viral transmission, infectivity, and COVID-19 severity are discussed. This review also addresses the effectiveness of human neutralizing antibodies induced by SARS-CoV-2 infection or immunization and the therapeutic antibodies against the ascended variants. Published March 30, 2024: https://doi.org/10.1007/s15010-024-02223-y

|
Scooped by
Juan Lama
|
Background The COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 virus, emerged in late 2019 and spready globally. Many effects of infection with this pathogen are still unknown, with both chronic and repeated COVID-19 infection producing novel pathologies. Case presentation An immunocompromised patient presented with chronic COVID-19 infection. The patient had history of Hodgkin’s lymphoma, treated with chemotherapy and stem cell transplant. During the course of their treatment, eleven respiratory samples from the patient were analyzed by whole-genome sequencing followed by lineage identification. Whole-genome sequencing of the virus present in the patient over time revealed that the patient at various timepoints harboured three different lineages of the virus. The patient was initially infected with the B.1.1.176 lineage before coinfection with BA.1. When the patient was coinfected with both B.1.1.176 and BA.1, the viral populations were found in approximately equal proportions within the patient based on sequencing read abundance. Upon further sampling, the lineage present within the patient during the final two timepoints was found to be BA.2.9. The patient eventually developed respiratory failure and died. Conclusions This case study shows an example of the changes that can happen within an immunocompromised patient who is infected with COVID-19 multiple times. Furthermore, this case demonstrates how simultaneous coinfection with two lineages of COVID-19 can lead to unclear lineage assignment by standard methods, which are resolved by further investigation. When analyzing chronic COVID-19 infection and reinfection cases, care must be taken to properly identify the lineages of the virus present. Key points -
A patient repeatedly tested positive for COVID-19 over 16 months. -
Infection progressed from one lineage to coinfection with a second lineage, before clearance of coinfection and reinfection with a third, different lineage. -
Coinfection was difficult to identify through genomic methods. Published in Virology Journal (Jan. 4, 2024): https://doi.org/10.1186/s12985-023-02278-7

|
Scooped by
Juan Lama
|
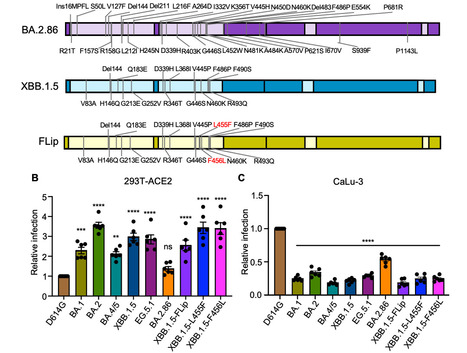
Evolution of SARS-CoV-2 requires the reassessment of current vaccine measures. Here, we characterized BA.2.86 and the XBB-lineage variant FLip by investigating their neutralization alongside D614G, BA.1, BA.2, BA.4/5, XBB.1.5, and EG.5.1 by sera from 3-dose vaccinated and bivalent vaccinated healthcare workers, XBB.1.5-wave infected first responders, and monoclonal antibody (mAb) S309. We assessed the biology of the variant Spikes by measuring viral infectivity and membrane fusogenicity. BA.2.86 is less immune evasive compared to FLip and other XBB variants, consistent with antigenic distances. Importantly, distinct from XBB variants, mAb S309 was unable to neutralize BA.2.86, likely due to a D339H mutation based on modeling. BA.2.86 had relatively high fusogenicity and infectivity in CaLu-3 cells but low fusion and infectivity in 293T-ACE2 cells compared to some XBB variants, suggesting a potentially differences conformational stability of BA.2.86 Spike. Overall, our study underscores the importance of SARS-CoV-2 variant surveillance and the need for updated COVID-19 vaccines. Preprint available in bioRxiv (Sept. 12, 2023): https://doi.org/10.1101/2023.09.11.557206

|
Scooped by
Juan Lama
|
EG.5.1 is a subvariant of the SARS-CoV-2 Omicron XBB variant that is rapidly increasing in prevalence worldwide. EG.5.1 has additional substitutions in its spike protein (namely, Q52H and F456L) compared with XBB.1.5. However, the pathogenicity, transmissibility, and immune evasion properties of clinical isolates of EG.5.1 are largely unknown. In this study, we used wild-type Syrian hamsters to investigate the replicative ability, pathogenicity, and transmissibility of a clinical EG.5.1 isolate. Our data show that there are no obvious differences in growth ability and pathogenicity between EG.5.1 and XBB.1.5, and both EG.5.1 and XBB.1.5 are attenuated compared to a Delta variant isolate. We also found that EG.5.1 is transmitted more efficiently between hamsters compared with XBB.1.5. In addition, unlike XBB.1.5, we detected EG.5.1 virus in the lungs of four of six exposed hamsters, suggesting that the virus tropism of EG.5.1 is different from that of XBB.1.5 after airborne transmission. Finally, we assessed the neutralizing ability of plasma from convalescent individuals and found that the neutralizing activity against EG.5.1 was slightly, but significantly, lower than that against XBB.1.5 or XBB.1.9.2. This suggests that EG.5.1 effectively evades humoral immunity and that the amino acid differences in the S protein of EG.5.1 compared with that of XBB.1.5 or XBB.1.9.2 (i.e., Q52H, R158G, and F456L) alter the antigenicity of EG.5.1. Our data suggest that the increased transmissibility and altered antigenicity of EG.5.1 may be driving its increasing prevalence over XBB.1.5 in the human population. Available as preprint in bioRxiv (Sept 1 2023): https://doi.org/10.1101/2023.08.31.555819

|
Scooped by
Juan Lama
|
Health risk of EG.5, which is related to Omicron subvariant, judged to be low but may drive larger wave of infections. A new strain of Covid-19 has been designated as a variant of interest by the World Health Organization, although the public health risk has been judged as low. The variant, known as EG.5 or “Eris”, is related to an Omicron subvariant called XBB.1.9.2, and is growing in prevalence globally, with countries including the UK, China and US among those affected. However, the WHO suggested the variant does not pose a particular threat. “Based on the available evidence, the public health risk posed by EG.5 is evaluated as low at the global level,” the agency said, adding that the risk appeared to be on a par with other circulating variants of interest. “While EG.5 has shown increased prevalence, growth advantage, and immune escape properties, there have been no reported changes in disease severity to date,” the WHO added....

|
Scooped by
Juan Lama
|
HIV BREAKTHROUGH - Genetic study identifies Africa-specific variant near CHD1L gene associated with lower HIV-1 viral load in populations of African descent. New research has found a genetic variant that may explain why some people of African ancestry have naturally lower viral loads of HIV, reducing their risk of transmitting the virus and slowing progress of their own illness. The paper, published today in Nature, demonstrates the first new genetic variant related to HIV infection discovered in nearly 30 years of research. It could, in the future, help direct the development of new treatments and approaches for those living with HIV. HIV-1 remains a significant global health crisis, and identifying new targets for therapies is crucial. The study focuses on individuals of African descent due to the disproportionate burden of HIV-1 in Africa and the high genetic diversity in the region. An international team of researchers analysed the DNA of almost 4,000 people of African ancestry living with HIV-1, the most common type of the virus. They identified a variant within a region on chromosome 1 containing the gene CHD1L which is associated with reduced viral load in carriers of the variant. Between 4% and 13 % of people of African origin are thought to carry this particular variant. Viral load is the amount of a virus in a patient’s system. Higher levels are known to correlate with faster disease progression and increased risk of transmission. But viral load varies widely among infected individuals, influenced by a number of factors including an individual’s genetic makeup. Co-Senior author Professor Manjinder Sandhu, Chair in Population Health and Data Science in the School of Public Health, said: “With more than a million new HIV infections a year, it’s clear that we still have a long way to go in the fight against HIV – we are yet to have a vaccine to prevent infection, have yet to find a cure and still see drug resistance emerging in some individuals. The next step is to fully understand exactly how this genetic variant controls HIV replication.” Understanding African populations Most of what we know about the relationship between our DNA and HIV comes from studies among European populations. But given that HIV disproportionately affects people on the African continent – more than 25 million people who are HIV-positive live on the continent – it’s important to better understand the role of genetics in HIV infection in African populations. Paul McLaren from the Public Health Agency of Canada’s National Microbiology Laboratory and joint first author on the paper, said: “African populations are still drastically underrepresented in human DNA studies, despite experiencing the highest burden of HIV infection. By studying a large sample of people of African ancestry, we’ve been able to identify a new genetic variant that only exists in this population and which is linked to lower HIV viral loads.” Reducing viral load CHD1L is known to play a role in repairing damaged DNA, though it is not clear why the variant should be important in reducing viral load. However, as HIV attacks immune cells, researchers at the University of Cambridge’s Department of Medicine, led by Dr Harriet Groom and Professor Andrew Lever, used stem cells to generate variants of cells that HIV can infect in which CHD1L had either been switched off or its activity turned down. HIV turned out to replicate better in a type of immune cell known as a macrophage when CHD1L was switched off. In another cell type, the T cell, there was no effect – perhaps surprising since most HIV replication occurs in the latter cell type. Dr Harriet Groom said: “This gene seems to be important to controlling viral load in people of African ancestry. Although we don’t yet know how it’s doing this, every time we discover something new about HIV control, we learn something new about the virus and something new about the cell. The link between HIV replication in macrophages and viral load is particularly interesting and unexpected. Original study published (August 2, 2023) in Nature: https://doi.org/10.1038/s41586-023-06370-4

|
Scooped by
Juan Lama
|
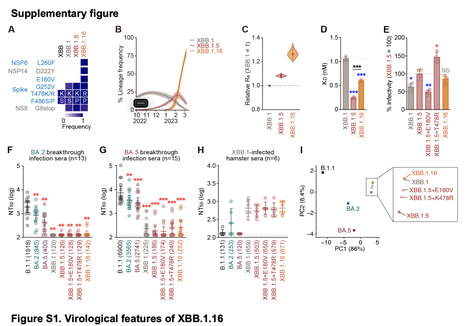
At the end of March 2023, XBB.1.16, a SARS-CoV-2 omicron XBB subvariant, emerged and was detected in various countries. Compared to XBB.1.5, XBB.1.16 has two substitutions in the S protein: E180V is in the N-terminal domain, and T478K in the receptor-binding domain (RBD). We first show that XBB.1.16 had an effective reproductive number (Re) that was 1.27- and 1.17-fold higher than the parental XBB.1 and XBB.1.5, respectively, suggesting that XBB.1.16 will spread worldwide in the near future. In fact, the WHO classified XBB.1.16 as a variant under monitoring on March 30, 2023. Neutralization assays demonstrated the robust resistance of XBB.1.16 to breakthrough infection sera of BA.2 (18-fold versus B.1.1) and BA.5 (37-fold versus B.1.1). We then used six clinically-available monoclonal antibodies and showed that only sotrovimab exhibits antiviral activity against XBB subvariants, including XBB.1.16. Our results suggest that, similar to XBB.1 and XBB.1.5, XBB.1.16 is robustly resistant to a variety of anti-SARS-CoV-2 antibodies. Our multiscale investigations suggest that XBB.1.16 that XBB.1.16 has a greater growth advantage in the human population compared to XBB.1 and XBB.1.5, while the ability of XBB.1.16 to exhibit profound immune evasion is comparable to XBB.1 and XBB.1.5. The increased fitness of XBB.1.16 may be due to (1) different antigenicity than XBB.1.5; and/or (2) the mutations in the non-S viral protein(s) that may contribute to increased viral growth efficiency. Published in bioRxiv (april 6, 2023): https://doi.org/10.1101/2023.04.06.535883

|
Scooped by
Juan Lama
|
Monoclonal antibodies (mAbs) offer a treatment option for individuals with severe COVID-19 and are especially important in high-risk individuals where vaccination is not an option. Given the importance of understanding the evolution of resistance to mAbs by SARS-CoV-2, we reviewed the available in vitro neutralization data for mAbs against live variants and viral constructs containing spike mutations of interest. Unfortunately, evasion of mAb-induced protection is being reported with new SARS-CoV-2 variants. The magnitude of neutralization reduction varied greatly among mAb–variant pairs. For example, sotrovimab retained its neutralization capacity against Omicron BA.1 but showed reduced efficacy against BA.2, BA.4 and BA.5, and BA.2.12.1. At present, only bebtelovimab has been reported to retain its efficacy against all SARS-CoV-2 variants considered here. Resistance to mAb neutralization was dominated by the action of epitope single amino acid substitutions in the spike protein. Although not all observed epitope mutations result in increased mAb evasion, amino acid substitutions at non-epitope positions and combinations of mutations also contribute to evasion of neutralization. This Review highlights the implications for the rational design of viral genomic surveillance and factors to consider for the development of novel mAb therapies. In this Review, Carabelli, Robertson and colleagues explore data on the neutralization of globally circulating variants of concern by monoclonal antibodies (mAbs) and discuss how knowledge of the dynamics of viral evasion of mAbs can contribute to viral surveillance and the development of novel mAb treatments, as well as inform predictions of resistance that may arise in the future. Published in Nature (October 28, 2022): https://doi.org/10.1038/s41579-022-00809-7

|
Scooped by
Juan Lama
|
The B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has splintered into multiple subvariants with increased transmissibility and immune escape.1 At the time of this report, omicron subvariant BA.5 is the dominant global virus and has shown substantial immune escape as compared with previous omicron subvariants.2-5 BA.4.6 is a sublineage of BA.4 with two additional mutations in the spike protein (R346T and N658S) (Figure 1A) and has recently increased in prevalence in certain regions currently dominated by BA.5, including in the United States. The ability of BA.4.6 to evade neutralizing antibodies that were induced by infection or vaccination remains to be determined. We evaluated neutralizing antibody titers against five SARS-CoV-2 strains — WA1/2020 and omicron subvariants BA.1, BA.2, BA.4–BA.5, and BA.4.6 — in 19 participants who had been recently infected with the omicron BA.1 or BA.2 subvariant and in 16 participants who had been vaccinated and boosted with the original mRNA-1273 vaccine (Moderna) (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). In the cohort with previous omicron infection, all the participants except for one had been vaccinated; samples were obtained a median of 21 days after diagnosis of omicron infection. In this cohort, the median pseudovirus neutralizing antibody titer was 42,067 against WA1/2020, 6352 against BA.1, 3854 against BA.2, 1673 against BA.4–BA.5, and 630 against BA.4.6 (Figure 1B). The median neutralizing antibody titers against BA.4.6 were lower than the median titers against WA1/2020 by a factor of 67, against BA.1 by a factor of 10, against BA.2 by a factor of 6, and against BA.4–BA.5 by a factor of 2.7. In the mRNA-1273 vaccine cohort, participants were excluded if they had a known history of SARS-CoV-2 infection or positive results on nucleocapsid serologic analysis or if they had received immunosuppressive medications or other vaccines against SARS-CoV-2. Six months after the initial two mRNA-1273 immunizations, the median neutralizing antibody titer was 951 against WA1/2020, 28 against BA.2, 30 against BA.4–BA.5, and 23 against BA.4.6 (Figure 1C). At a median of 17 days after the first booster dose, the median neutralizing antibody titer was 16,011 against WA1/2020, 802 against BA.2, 449 against BA.4–BA.5, and 225 against BA.4.6. The median neutralizing antibody titer against BA.4.6 was lower than that against WA1/2020 by a factor of 71, against BA.2 by a factor of 4, and against BA.4–BA.5 by a factor of 2. Our data show that the BA.4.6 omicron subvariant markedly escaped neutralizing antibodies induced by infection or vaccination, with values that were lower than BA.5 titers by a factor of 2 to 2.7, which suggests continued evolution of SARS-CoV-2. These findings provide immunologic context for the increasing prevalence of BA.4.6 in populations in which BA.5 is currently dominant. Moreover, the R346T mutation had also recently been observed in other omicron subvariants, including BA.2.75 and BA.5, which suggests the biologic relevance of this mutation. The potential effect of the emergence of the BA.4.6 subvariant on vaccine boosters containing BA.5 immunogens or on infection with BA.5 remains to be determined. Published in NEJM (Oct. 19, 2022): https://doi.org/10.1056/NEJMc2212117

|
Scooped by
Juan Lama
|
The XBB COVID subvariant combines Omicron strains and is driving cases in Singapore and Bangladesh. New variants of the rapidly mutating coronavirus are still popping up around the world, and a new iteration of COVID on the rise in Asia may be the most immune-evasive yet. The XBB strain is causing a small surge in cases in countries like Bangladesh and Singapore. The latter has recorded a daily average of about 5,500 cases over the past week, compared to a daily average of 2,000 cases a month ago. Officials in Singapore, which is scaling back its last remaining COVID restrictions to reopen to the rest of the world, are not yet concerned about the increase in cases. On Monday, Health Minister Ong Ye Kung noted that only 15% of the country’s recent COVID cases were reinfections. “If you start to see 50% getting it a second time, you’re going to have a wave,” he said. The health ministry did start doling out Moderna’s Omicron booster on Tuesday, three days ahead of schedule, citing rising infections caused by the new “XBB Omicron subvariant.” Hospitalizations in Singapore have increased alongside the rise in cases, yet deaths remain low, with fewer than a dozen recorded in the country over the past week. Over 90% of Singapore’s population has received two doses of a COVID vaccine, and 79% have received at least one booster. Bangladesh is also reporting a small uptick in cases, though reported numbers are smaller than Singapore’s. The South Asian country reported a daily average of about 500 cases during the week of Oct. 3, compared to an average of 300 cases a month earlier. Bangladesh has a vaccination rate of 75.5%. On Monday, Hong Kong’s health authorities said they found the city’s first case of XBB.1, a subvariant of the XBB strain, in an imported case from the U.S. Officials said the positive case showed no symptoms. Immune evasive Experts are paying close attention to the XBB strain, which combines two different Omicron strains. Eric Topol, founder of the the Scripps Research Translational Institute, tweeted that XBB is one “of the most important variants [to] watch right now.” The new strain is “probably the most immune-evasive yet” due to its combination of mutations from other strains, Raj Rajnarayanan, a professor at the New York Institute of Technology’s Jonesboro, Ark., campus, told Fortune in September. A preprint study from Oct. 4, authored by researchers at Peking University and Changping Laboratory, found that XBB had the greatest ability to evade antibody protections among newly emerging variants. Experts are also concerned that monoclonal antibody treatments might be less effective against newer variants like XBB and BA.2.75.2. “We’ve not seen this type of immune evasion before,” Michael Osterholm, director of the University of Minnesota’s Center for Infectious Disease Research and Policy (CIDRAP), told Fortune earlier this month. New variants BA.5 is still the most dominant strain of COVID in the U.S., making up 79.2% of COVID cases recorded between Oct. 2 and Oct. 11, according to the Centers for Disease Control and Prevention’s Nowcast. But other variants are starting to catch up. Over the past week, 13.6% of cases were from the BA.4.6 subvariant, an increase from 6.4% of cases two months ago. The BF.7 strain is also spreading quickly, making up 4.6% of cases today compared to just 0.3% two months ago. In order to keep ahead of the rapidly mutating coronavirus, the U.S. approved bivalent boosters targeting the BA.4 and BA.5 subvariants on Aug. 31. Yet only 7.6 million Americans have received the Omicron booster since it became widely available on Labor Day. A survey from the Kaiser Family Foundation found that two-thirds of Americans are either putting off getting the new booster or saying they won’t get it at all.

|
Scooped by
Juan Lama
|
In this study, by characterizing several human monoclonal antibodies (mAbs) isolated from single B cells of the COVID-19–recovered individuals in India who experienced ancestral Wuhan strain (WA.1) of SARS-CoV-2 during early stages of the pandemic, we found a receptor binding domain (RBD)–specific mAb 002-S21F2 that has rare gene usage and potently neutralized live viral isolates of SARS-CoV-2 variants including Alpha, Beta, Gamma, Delta, and Omicron sublineages (BA.1, BA.2, BA.2.12.1, BA.4, and BA.5) with IC50 ranging from 0.02 to 0.13 μg/ml. Structural studies of 002-S21F2 in complex with spike trimers of Omicron and WA.1 showed that it targets a conformationally conserved epitope on the outer face of RBD (class 3 surface) outside the ACE2-binding motif, thereby providing a mechanistic insights for its broad neutralization activity. The discovery of 002-S21F2 and the broadly neutralizing epitope it targets have timely implications for developing a broad range of therapeutic and vaccine interventions against SARS-CoV-2 variants including Omicron sublineages. Published in Science Advances (October 5, 2022): https://doi.org/10.1126/sciadv.add2032

|
Scooped by
Juan Lama
|
Infection with a pre-Omicron SARS-CoV-2 variant protects against reinfection with a second, although the effect fades almost completely after three years. Natural immunity induced by infection with SARS-CoV-2 provides a strong shield against reinfection by a pre-Omicron variant for 16 months or longer, according to a study1. This protection against catching the virus dwindles over time, but immunity triggered by previous infection also thwarts the development of severe COVID-19 symptoms — and this safeguard shows no signs of waning. The study1, which analyses cases in the entire population of Qatar, suggests that although the world will continue to be hit by waves of SARS-CoV-2 infection, future surges will not leave hospitals overcrowded with people with COVID-19. The research was posted on the medRxiv preprint server on 7 July. It has not yet been peer reviewed. The study is “solid”, says Shane Crotty, an immunologist at the La Jolla Institute for Immunology in California. “The data make sense and are in line with multiple other studies and previous work by this group.” Better late than never But scientists also warn that the study’s results do not mean that infected people can skip vaccination. A separate study2 by many of the same authors found that “people who had both natural immunity and vaccine immunity were substantially more protected against the virus than people who had only natural immunity alone or vaccine immunity”, says Laith Abu-Raddad, an infectious-disease epidemiologist at Weill Cornell Medicine–Qatar in Doha and a co-author of both studies. “It was very clear-cut.” Studies3,4 on the effectiveness of COVID-19 vaccines suggest that protection against the virus SARS-CoV-2 decreases over time, waning considerably after six months. To learn about the course of naturally acquired immunity, the authors examined COVID-19 data gathered in Qatar between 28 February 2020 and 5 June 2022. “Our study is the first to have such a long time of follow-up,” says co-author Hiam Chemaitelly, an epidemiologist also at Weill Cornell Medicine–Qatar. The researchers compared COVID-19 cases in unvaccinated individuals who’d had one previous SARS-CoV-2 infection with cases in unvaccinated people who’d never previously caught the virus. They found that infection with a pre-Omicron variant prevented reinfection by another pre-Omicron variant with an average effectiveness of 85.5% for the period covering the 4th through the 16th month following the initial infection. Effectiveness peaked at 90.5% in the 7th month after the first infection and fell to about 70% at 16 months (see ‘Immunity fades away’). By extrapolating this trend, the authors predict that effectiveness against reinfection will fall to less than 10% 32 months after the first infection. Pre-Omicron infection was only 38% effective at preventing infection by an Omicron variant in the first 6 months after Omicron emerged. Modelling suggests that the number will drop to 10% at 15 months. All the same, infection with any SARS-CoV-2 variant is highly effective at combating severe, critical or fatal COVID-19 after reinfection: effectiveness was around 100% up to the 14th month after primary infection and showed no signs of declining. Old and young alike The authors note that most of Qatar’s population is young, so the findings might not apply to populations with a higher average age. But when the team restricted its analysis to people more than 50 years old, the levels of protection were similar. Other potential caveats exist. The authors’ projections assume that the immune response changes at a specific rate, when in fact that rate depends on the length of time since a person caught the virus, says Crotty. Therefore, immune responses measured at one point in time might not allow accurate predictions of the future. Regardless of the extrapolations, the data indicate that naturally acquired immunity is hardy — something that is not always championed. “In the US, we were underselling the immune protection provided by previous infection,” says Jeffrey Morris, a biomedical data scientist at the University of Pennsylvania in Philadelphia. He adds that the Qatar team’s study affirms the substantial evidence for natural immunity’s capabilities. Published in Nature July 15, 2022 https://doi.org/10.1038/d41586-022-01914-6
|



 Your new post is loading...
Your new post is loading...