Your new post is loading...

|
Scooped by
Juan Lama
|
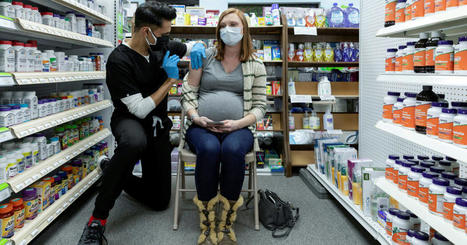
In an early analysis of coronavirus vaccine safety data, researchers at the Centers for Disease Control and Prevention have found no evidence that the Pfizer-BioNTech or Moderna vaccines pose serious risks during pregnancy. The findings are preliminary and cover just the first 11 weeks of the U.S. vaccination program. But the study, which included self-reported data on more than 35,000 people who received one of the vaccines during or shortly before pregnancy, is the largest yet on the safety of the coronavirus vaccines in pregnant people. During the clinical trials of the vaccines, pregnant women were excluded. That left patients, doctors and experts unsure whether the shots were safe to administer during pregnancy. “There’s a lot of anxiety about whether it’s safe and whether it would work and what to expect as far as side effects,” said Dr. Stephanie Gaw, a maternal-fetal medicine specialist at the University of California, San Francisco. The new data, Dr. Gaw said, demonstrate that “a lot of pregnant people are getting the vaccine, there isn’t a significant increase in adverse pregnancy effects at this point, and that side effect profiles are very similar to nonpregnant people.” “I think that’s all very reassuring,” she said, “and I think it will really help providers and public health officials more strongly recommend getting the vaccine in pregnancy.” Covid-19 poses serious risks during pregnancy. Pregnant women who develop symptoms of the disease are more likely to become seriously ill, and more likely to die, than nonpregnant women with symptoms.Beca use of those risks, the C.D.C. has recommended that coronavirus vaccines be made available to pregnant women, though it also suggests that they consult with their doctors when making a decision about vaccination. The new study, which was published on Wednesday in The New England Journal of Medicine, is based largely on self-reported data from V-safe, the C.D.C.’s coronavirus vaccine safety monitoring system. Participants in the program use a smartphone app to complete regular surveys about their health, and any side effects they might be experiencing, after receiving a Covid-19 vaccine. The researchers analyzed the side effects reported by V-safe participants who received either the Pfizer or Moderna vaccine between Dec. 14, 2020, and Feb. 28, 2021. They focused on 35,691 participants who said that they had been pregnant when they received the vaccine or became pregnant shortly thereafter. After vaccination, pregnant participants reported the same general pattern of side effects that nonpregnant ones did, the researchers found: pain at the injection site, fatigue, headaches and muscle pain. Women who were pregnant were slightly more likely to report injection site pain than women who were not, but less likely to report the other side effects. They were also slightly more likely to report nausea or vomiting after the second dose. Pregnant V-safe participants were also given an opportunity to enroll in a special registry that tracked pregnancy and infant outcomes. By the end of February, 827 of those enrolled in the pregnancy registry had completed their pregnancies, 86 percent of which resulted in a live birth. Rates of miscarriage, prematurity, low birth weight and birth defects were consistent with those reported in pregnant women before the pandemic, the researchers report. “This study is of critical importance to pregnant individuals,” Dr. Michal Elovitz, a maternal-fetal medicine specialist at the University of Pennsylvania, said in an email. “It is very reassuring that there were no reported acute events in pregnant individuals” over the course of the study, she said. But the report has several limitations and much more research is needed, experts said. Enrollment in the surveillance programs is voluntary and the data are self-reported. In addition, because the study period encompassed just the first few months of the U.S. vaccination campaign, the vast majority of those enrolled in the pregnancy registry were health care workers. And there is not yet any data on pregnancy outcomes from people who were vaccinated during the first trimester of pregnancy. “I think we can feel more confident about recommending the vaccine in pregnancy, and especially with pregnant people that are at risk of Covid,” Dr. Gaw said. “But we do need to wait for more data for complete pregnancy outcomes from vaccines early in pregnancy.” Findings published in NEJM (April 21, 2021): https://doi.org/10.1056/NEJMoa2104983

|
Scooped by
Juan Lama
|
A link to the vaccines is not certain, and investigations are underway in some reported cases. One day after receiving her first dose of Moderna’s Covid vaccine, Luz Legaspi, 72, woke up with bruises on her arms and legs, and blisters that bled inside her mouth. She was hospitalized in New York City that day, Jan. 19, with a severe case of immune thrombocytopenia — a lack of platelets, a blood component essential for clotting. The same condition led to the death in January of Dr. Gregory Michael, 56, an obstetrician in Miami Beach whose symptoms appeared three days after he received the Pfizer-BioNTech vaccine. Treatments failed to restore his platelets, and after two weeks in the hospital he died from a brain hemorrhage. It is not known whether this blood disorder is related to the Covid vaccines. More than 31 million people in the United States have received at least one dose, and 36 similar cases had been reported to the government’s Vaccine Adverse Event Reporting System, VAERS, by the end of January. The cases involved either the Pfizer-BioNTech or Moderna vaccine, the only two authorized so far for emergency use in the United States. But the reporting system shows only problems described by health care providers or patients after vaccination, and does not indicate whether the shots actually caused the problems. Officials with the Food and Drug Administration and the Centers for Disease Control and Prevention said that they were looking into the reports, but that so far, rates of the condition in vaccinated people did not appear higher than the rates normally found in the U.S. population, so the cases could be coincidental. Overall, the vaccines are considered safe. A small number of severe allergic reactions have been reported, but they are treatable, and the rates are in line with those reported for other vaccines, regulators say. In a statement, Pfizer said: “We take reports of adverse events very seriously,” and added that it was aware of thrombocytopenia cases in vaccine recipients. The statement also said: “We are collecting relevant information to share with the F.D.A. However, at this time, we have not been able to establish a causal association with our vaccine.” Moderna also provided a statement, which did not address the question of the platelet disorder, but said the company “continuously monitors the safety of the Moderna Covid-19 vaccine using all sources of data” and routinely shares safety information with regulators. Hematologists with expertise in treating immune thrombocytopenia said they suspected that the vaccine did play a role. But they said that cases after vaccination were likely to be exceedingly rare, possibly the result of an unknown predisposition in some people to react to the vaccine by developing an immune response that destroys their platelets. The disorder has occurred, rarely, in people who received other inoculations, particularly the measles-mumps-rubella one. “I think it is possible that there is an association,” Dr. James Bussel, a hematologist and professor emeritus at Weill Cornell Medicine who has written more than 300 scientific articles on the platelet disorder, said in an interview. “I’m assuming there’s something that made the people who developed thrombocytopenia susceptible, given what a tiny percentage of recipients they are.” He added: “Having it happen after a vaccine is well-known and has been seen with many other vaccines. Why it happens, we don’t know.” Dr. Bussel said it was important to share information about the cases, because severe thrombocytopenia can be serious, and physicians need to know how to treat it. Sometimes the condition resists standard therapies, and if very low platelet counts persist, the patient faces an increasing risk of severe bleeding and even brain hemorrhage. He and a colleague, Dr. Eun-Ju Lee, have submitted an article to a medical journal on 15 cases in Covid vaccine recipients they identified by searching the government’s database or by consulting with other physicians treating patients. The report provides information about treatments and urges doctors to report cases. It also notes that it is too soon to tell whether the affected patients will have lasting recoveries, or recurrences of the platelet problem. A few of the patients had previously had platelet disorders or other autoimmune conditions that might have made them vulnerable, Dr. Bussel (pronounced Bew-SELL) said. People can have low platelets without symptoms, and it is possible that in some, a vaccine reaction could drop the level further, to a point where it becomes apparent by causing bruises or bleeding, Dr. Bussel said....

|
Scooped by
Juan Lama
|
Anaphylaxis was rarely reported among the first 4 million recipients of the second COVID-19 vaccine authorized for use in the United States, the CDC and FDA reported in MMWR. According to the report, between Dec. 21 and Jan. 10, more than 1,200 adverse events were reported following vaccination with Moderna’s COVID-19 vaccine. Of these, 108 were determined to be allergic reactions and 10 were confirmed as cases of anaphylaxis — equating to 2.5 cases per million doses administered — according to data compiled from the Vaccine Adverse Event Reporting System. The report outlined that nine of the cases of anaphylaxis were reported among people with a documented history of allergies or allergic reactions, including five with a history of anaphylaxis. According to the report, the median time between vaccine receipt to symptom onset was 7.5 minutes but ranged from anywhere between 1 and 45 minutes. Of the eight individuals with follow-up information available, all reportedly recovered or had been discharged home. “Based on this early monitoring, anaphylaxis after receipt of Moderna COVID-19 vaccine appears to be a rare event; however, comparisons of anaphylaxis risk with that associated with non–COVID-19 vaccines are constrained at this time by the limited data available this early in the COVID-19 vaccination program,” members of the CDC COVID-19 Response Team and the FDA wrote in the report. Moderna’s vaccine was the second COVID-19 shot authorized for emergency use in the U.S. Earlier this month, the CDC and FDA reported that the first vaccine, developed by Pfizer and BioNTech COVID-19, also was rarely associated with anaphylaxis, with 21 cases of confirmed among the first 1,893,360 recipients of the vaccine. At the time, Nancy E. Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, said the number “may seem high, but this is still a rare outcome.” As a precaution, researchers said that vaccination sites should be outfitted to handle these reactions should they arise, and the CDC has issued updated interim clinical considerations to reflect that. “Locations administering COVID-19 vaccines should adhere to CDC guidance, including screening recipients for contraindications and precautions, having necessary supplies and staff members available to manage anaphylaxis, implementing recommended postvaccination observation periods, and immediately treating suspected anaphylaxis with intramuscular epinephrine injection,” the authors of the new report wrote. CDC report (Jan.22, 2021) available at:
|

|
Scooped by
Juan Lama
|
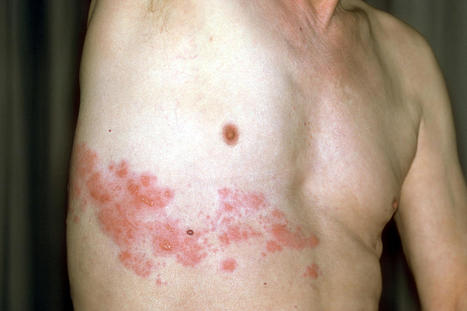
Herpes infections may be a side effect of the COVID-19 vaccine, experts have revealed. Scientists in Israel identified six cases in a new study of patients developing a skin rash known as herpes zoster after receiving the Pfizer vaccine, according to a study in the Rheumatology journal. Herpes zoster starts off as a small, itchy skin rash, but if left untreated, it could cause nerve damage and pain, the Jerusalem Post reported. This can include a prolonged burning sensation on the skin even after the rash disappears. Researchers from Tel Aviv Sourasky Medical Center and Carmel Medical Center in Haifa found those with autoimmune inflammatory rheumatic diseases had a higher risk of developing the herpes. Out of 491 patients, six people or 1.2 percent experienced the infection, researchers said. The six patients all have mild cases of autoimmune inflammatory rheumatic diseases and were young, though the infection is generally more common in those over the age of 50. “That is why we reported on it,” Dr. Victoria Furer, the lead author, told the outlet. Five of them developed herpes zoster after the first dose and the sixth got it after the second. But it’s still unclear whether the vaccine caused the cases of herpes zoster. “We cannot say the vaccine is the cause at this point,” Furer told the outlet. “We can say it might be a trigger in some patients.” “We should not scare people,” she told the Jerusalem Post. “The overall message is to get vaccinated. It is just important to be aware.” Original research published in Rheumathology (April 12, 2021): https://doi.org/10.1093/rheumatology/keab345

|
Scooped by
Juan Lama
|
The Prime Minister of Norway, Erna Solberg, says her country may fine-tune the vaccination of its oldest, sickest citizens as it tries to make sense of a recent spate of deaths. Having weathered the pandemic better than most, Norway suddenly made international headlines this month after revealing that more than 30 people -- all over 70 and all already sick -- died not long after being vaccinated against Covid-19. Solberg says the intense global interest in the news was “exaggerated” as she tries to ensure the development doesn’t put people off inoculation. “We don’t believe there’s any problem with the safety of the vaccines,” Solberg said in an interview with Bloomberg Live that aired on Tuesday. “But we will maybe not give them to the most vulnerable of the elderly, because that might speed up a process where they were what we would say at the end of life phase anyway,” so, “that probably is not what we will continue to do.” The Norwegian Institute of Public Health, which has identified people over 65 as a group to be prioritized in the vaccine rollout, has urged doctors to inoculate the elderly and sick on a case-by-case basis. “For very frail patients and terminally ill patients, a careful balancing of benefit versus disadvantage of vaccination is recommended,” it said on Jan. 11, before Norway published data on post-inoculation deaths. The Norwegian Medicines Agency said its reporting on adverse reactions caused unnecessary concern around the world, and will now only publish data on deaths that have been autopsied. The agency says its information on the side-effects of Covid-19 vaccines will be included in international studies. Other countries, including Germany, have also recorded deaths in people who recently were vaccinated. Finland reported three such fatalities on Tuesday, but none of the countries identified causal links. The Norwegian Medicines Agency says there’s no evidence so far that the elderly deaths reported were directly linked to the vaccine. “However, it cannot be ruled out that common vaccine side effects, such as fever and nausea, may have contributed to a serious course of underlying disease in frail patients,” the agency said in a written response to questions. Norway is now working with Pfizer Inc. and BioNTech SE -- the first producers to provide it with vaccines -- to examine its data in more detail. The country’s medicines agency has told Pfizer it doesn’t see grounds for alarm. The first Europe-wide safety report on the Pfizer-BioNTech vaccine is due to be published at the end of January. Meanwhile, Norway’s government has underscored its confidence in the vaccine. “We are trying to work very hard to get the focus that this is not a problem,” Solberg said. “It’s who we have vaccinated, not the vaccine that has created this data.” Norway’s medicines agency reported 292 suspected “adverse reactions” out of 71,971 people who had been vaccinated as of Jan. 21; of those, 104 had been reviewed by health authorities, with 30 reported deaths. The country had inoculated 1.4% of its population as of Friday, according to Bloomberg’s Covid-19 Vaccine Tracker. That compares with 3.4% in neighboring Denmark, which is among the furthest advanced in Europe with its immunization program. Norway plans to administer second vaccine doses without delay, Solberg said. That’s in contrast to the approach in the U.K., where Health Secretary Matt Hancock has said there’s high confidence the first dose provides “decent efficacy” against the virus. Solberg spoke with Bloomberg Live before Norway moved to lock down the Oslo area in an effort to fight the spread of more contagious coronavirus mutations, deploying its strictest measures yet. The prime minister said she hopes Norway will finish vaccinating its most vulnerable citizens by March.
|



 Your new post is loading...
Your new post is loading...