Your new post is loading...

|
Scooped by
Juan Lama
|
Although ocular manifestations are reported in patients with COVID-19, consensus on ocular tropism of SARS-CoV-2 is lacking. Here, we infect K18-hACE2 transgenic mice with SARS-CoV-2 using various routes. We observe ocular manifestation and retinal inflammation with production of pro-inflammatory cytokines in the eyes of intranasally (IN)-infected mice. Intratracheal (IT) infection results in dissemination of the virus from the lungs to the brain and eyes via trigeminal and optic nerves. Ocular and neuronal invasions are confirmed using intracerebral (IC) infection. Notably, the eye-dropped (ED) virus does not cause lung infection and becomes undetectable with time. Ocular and neurotropic distribution of the virus in vivo is evident in fluorescence imaging with an infectious clone of SARS-CoV-2-mCherry. The ocular tropic and neuroinvasive characteristics of SARS-CoV-2 are confirmed in wild-type Syrian hamsters. Our data can improve the understanding regarding viral transmission and clinical characteristics of SARS-CoV-2 and help in improving COVID-19 control procedures. Published in Nature Comm. (Dec. 12, 2022): https://doi.org/10.1038/s41467-022-35225-1

|
Scooped by
Juan Lama
|
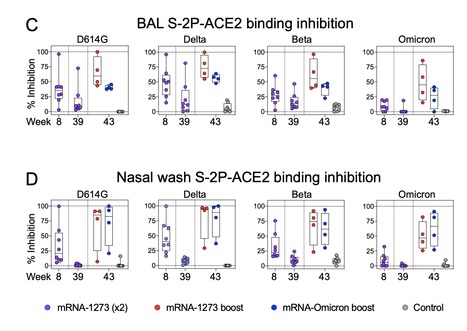
SARS-CoV-2 Omicron is highly transmissible and has substantial resistance to antibody neutralization following immunization with ancestral spike-matched vaccines. It is unclear whether boosting with Omicron-specific vaccines would enhance immunity and protection. Here, nonhuman primates that received mRNA-1273 at weeks 0 and 4 were boosted at week 41 with mRNA-1273 or mRNA-Omicron. Neutralizing antibody titers against D614G were 4760 and 270 reciprocal ID50 at week 6 (peak) and week 41 (pre-boost), respectively, and 320 and 110 for Omicron. Two weeks after boost, titers against D614G and Omicron increased to 5360 and 2980, respectively, for mRNA-1273 and 2670 and 1930 for mRNA-Omicron. Following either boost, 70-80% of spike-specific B cells were cross-reactive against both WA1 and Omicron. Significant and equivalent control of virus replication in lower airways was observed following either boost. Therefore, an Omicron boost may not provide greater immunity or protection compared to a boost with the current mRNA-1273 vaccine. Preprint available in bioRxix (Feb.04, 2022): https://doi.org/10.1101/2022.02.03.479037

|
Scooped by
Juan Lama
|
Scientists have created a first-of-its-kind mouse model of the COVID-19 disease, capable of revealing how the SARS-CoV-2 virus wreaks havoc on multiple organ systems in the animal's body. While this experimental model doesn't directly correspond to human coronavirus cases, it's a breakthrough in other ways, giving us a potential test system for exploring how the deadly respiratory pathogen extends way beyond the respiratory system in many cases of infection. "This mouse model is a really powerful tool for studying SARS-CoV-2 in a living system," explains cardiologist Arjun Deb from UCLA. Mouse models of COVID-19 have been engineered before, but none have gotten us to this point, the researchers say, by demonstrating what extra-pulmonary manifestations of COVID-19 look like. That's been a significant limitation for animal-based research into the virus's progression, and it's due to a couple of reasons. While mouse cells contain an analogue of the ACE2 receptor that the SARS-CoV-2 uses to bind to human cells, the virus doesn't seem to attach to the mouse version of the molecule. Genetically engineering mice with the human version of ACE2 provides a workaround of sorts, but before now, scientists hadn't succeeded in inducing multiple organ failure in mice, which could mimic the way human cases of extra-pulmonary infection tend to present. The shortfall may have been because prior studies used nasal inoculation on mice, infecting the animals with SARS-CoV-2 through the nose, which doesn't seem to develop into full-blown, system-wide coronavirus infections in mice. In the new study, led by first author Shen Li, a cardiologist at UCLA, the team got around this by injecting the virus into the bloodstreams of engineered mice, where it could reach the human version of ACE2 (called hACE2) in the heart and other vital organs. Unfortunately for the subjects, the experimental tweaks worked. And while it wasn't a good outcome for the mice involved, being able to study systemically induced toxicity in SARS-CoV-2-infected mice could significantly expand our knowledge of the virus's branching capabilities in human cases. Such knowledge is sorely needed. "Among COVID-19 patients, those who have organs involved other than the lungs are most at risk of a bad outcome," Deb says. "So we felt it was really important to understand how the virus affects those other organs." In the experiment, systemic administration of the infection provoked rapid results. Within seven days, the infected mice "demonstrated profound morbidity, severely restricted activity, and were found huddled at the corner of the cage", in contrast with a control group of similarly engineered mice who were spared the infection, remaining healthy. In the same amount of time, the infected mice lost up to about 25 percent of their body weight due to sharply reduced food consumption, necessitating euthanisation. The infected mice also had damaged spleens, irregular heart activity and blood pressure, and altered levels of immune cells – all symptoms resembling human cases of COVID-19. After the animals were euthanised, analysis of their organs revealed changes in gene expression in multiple tissues, disrupting cellular processes that generate energy in the body. "If a virus snuffs out the energy-generating pathways in multiple organs of the body, that's going to really wreak havoc," Deb explains. Beyond these effects, the infected mice also bore numerous signs of epigenetic changes, which could explain the altered gene expression evident in multiple organs. It's not known for sure, but the impacts of this could potentially be felt long after an infection has been beaten by the immune system – and, hypothetically speaking, could be the basis of the prolonged symptoms experienced by COVID-19 'long haulers'. "Although the physiological significance of SARS-CoV-2 altered DNA methylation patterns is not clear from our study, our model provides proof of concept that such epigenetic changes do occur soon after SARS-CoV-2 infection and can potentially led to persistent transcriptional changes affecting tissue homeostasis and organ function," the authors write in their paper. "Such epigenetic changes potentially occurring in humans with COVID-19 could lead to symptoms from persistent changes in dysregulated gene expression in infected tissues even in the absence of tissue viral burden."... Cited Research Published in JCI Insight (Dec. 7, 2020): https://doi.org/10.1172/jci.insight.145027
|

|
Scooped by
Juan Lama
|
The host response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can result in prolonged pathologies collectively referred to as post-acute sequalae of COVID-19 (PASC) or long COVID. To better understand the mechanism underlying long COVID biology, we compared the short- and long-term systemic responses in the golden hamster following either SARS-CoV-2 or influenza A virus (IAV) infection. Results demonstrated that SARS-CoV-2 exceeded IAV in its capacity to cause permanent injury to the lung and kidney and uniquely impacted the olfactory bulb (OB) and epithelium (OE). Despite a lack of detectable infectious virus, the OB and OE demonstrated myeloid and T cell activation, proinflammatory cytokine production, and an interferon response that correlated with behavioral changes extending a month post viral clearance. These sustained transcriptional changes could also be corroborated from tissue isolated from individuals who recovered from COVID-19. These data highlight a molecular mechanism for persistent COVID-19 symptomology and provide a small animal model to explore future therapeutics. Published in Science Transl. Med. (June 7, 2022): https://doi.org/10.1126/scitranslmed.abq3059

|
Scooped by
Juan Lama
|
Compared with earlier variants, Omicron may cause less damage to the lungs, new animal research suggests. A spate of new studies on lab animals and human tissues are providing the first hints of why the Omicron variant causes milder disease than previous versions of the coronavirus. In studies on mice and hamsters, Omicron produced less damaging infections, often limited largely to the upper airway: the nose, throat and windpipe. The variant did much less harm to the lungs, where previous variants would often cause scarring and serious breathing difficulty. “It’s fair to say that the idea of a disease that manifests itself primarily in the upper respiratory system is emerging,” said Roland Eils, a computational biologist at the Berlin Institute of Health, who has studiedhow coronaviruses infect the airway. In November, when the first report on the Omicron variant came out of South Africa, scientists could only guess at how it might behave differently from earlier forms of the virus. All they knew was that it had a distinctive and alarming combination of more than 50 genetic mutations. Previous research had shown that some of these mutations enabled coronaviruses to grab onto cells more tightly. Others allowed the virus to evade antibodies, which serve as an early line of defense against infection. But how the new variant might behave inside of the body was a mystery. “You can’t predict the behavior of virus from just the mutations,” said Ravindra Gupta, a virologist at the University of Cambridge. Over the past month, more than a dozen research groups, including Dr. Gupta’s, have been observing the new pathogen in the lab, infecting cells in Petri dishes with Omicron and spraying the virus into the noses of animals. As they worked, Omicron surged across the planet, readily infecting even people who were vaccinated or had recovered from infections. But as cases skyrocketed, hospitalizations increased only modestly. Early studies of patients suggested that Omicron was less likely to cause severe illness than other variants, especially in vaccinated people. Still, those findings came with a lot of caveats. For one thing, the bulk of early Omicron infections were in young people, who are less likely to get seriously ill with all versions of the virus. And many of those early cases were happening in people with some immunity from previous infections or vaccines. It was unclear whether Omicron would also prove less severe in an unvaccinated older person, for example. Experiments on animals can help clear up these ambiguities, because scientists can test Omicron on identical animals living in identical conditions. More than half a dozen experiments made public in recent days all pointed to the same conclusion: Omicron is milder than Delta and other earlier versions of the virus. On Wednesday, a large consortium of Japanese and American scientists released a report on hamsters and mice that had been infected with either Omicron or one of several earlier variants. Those infected with Omicron had less lung damage, lost less weight and were less likely to die, the study found. Although the animals infected with Omicron on average experienced much milder symptoms, the scientists were particularly struck by the results in Syrian hamsters, a species known to get severely ill with all previous versions of the virus. “This was surprising, since every other variant has robustly infected these hamsters,” said Dr. Michael Diamond, a virologist at Washington University and a co-author of the study. Several other studies on mice and hamsters have reached the same conclusion. (Like most urgent Omicron research, these studies have been posted online but have not yet been published in scientific journals.) The reason that Omicron is milder may be a matter of anatomy. Dr. Diamond and his colleagues found that the level of Omicron in the noses of the hamsters was the same as in animals infected with an earlier form of the coronavirus. But Omicron levels in the lungs were one-tenth or less of the level of other variants. A similar finding came from researchers at the University of Hong Kong who studied bits of tissue taken from human airways during surgery. In 12 lung samples, the researchers found that Omicron grew more slowly than Delta and other variants did. The researchers also infected tissue from the bronchi, the tubes in the upper chest that deliver air from the windpipe to the lungs. And inside of those bronchial cells, in the first two days after an infection, Omicron grew faster than Delta or the original coronavirus did. These findings will have to be followed up with further studies, such as experiments with monkeys or examination of the airways of people infected with Omicron. If the results hold up to scrutiny, they might explain why people infected with Omicron seem less likely to be hospitalized than those with Delta. Coronavirus infections start in the nose or possibly the mouth and spread down the throat. Mild infections don’t get much further than that. But when the coronavirus reaches the lungs, it can do serious damage. Immune cells in the lungs can overreact, killing off not just infected cells but uninfected ones. They can produce runaway inflammation, scarring the lung’s delicate walls. What’s more, the viruses can escape from the damaged lungs into the bloodstream, triggering clots and ravaging other organs. Dr. Gupta suspects that his team’s new data give a molecular explanation for why Omicron doesn’t fare so well in the lungs. Many cells in the lung carry a protein called TMPRSS2 on their surface that can inadvertently help passing viruses gain entry to the cell. But Dr. Gupta’s team found that this protein doesn’t grab on to Omicron very well. As a result, Omicron does a worse job of infecting cells in this manner than Delta does. A team at the University of Glasgow independently came to the same conclusion. Through an alternative route, coronaviruses can also slip into cells that don’t make TMPRSS2. Higher in the airway, cells tend not to carry the protein, which might explain the evidence that Omicron is found there more often than the lungs. Dr. Gupta speculated that Omicron evolved into an upper-airway specialist, thriving in the throat and nose. If that’s true, the virus might have a better chance of getting expelled in tiny drops into the surrounding air and encountering new hosts. “It’s all about what happens in the upper airway for it to transmit, right?” he said. “It’s not really what happens down below in the lungs, where the severe disease stuff happens. So you can understand why the virus has evolved in this way.” While these studies clearly help explain why Omicron causes milder disease, they don’t yet answer why the variant is so good at spreading from one person to another. The United States logged more than 580,000 cases on Thursday alone, the majority of which are thought to be Omicron. “These studies address the question about what may happen in the lungs but don’t really address the question of transmissibility,” said Sara Cherry, a virologist at the Perelman School of Medicine at the University of Pennsylvania. Dr. Diamond said he wanted to wait for more studies to be carried out, especially in people instead of animals, before endorsing the hypothesis that TMPRSS2 is the key to understanding Omicron. “I think it is still premature on this,” he said. Scientists know that part of Omicron’s contagiousness comes from its ability to evade antibodies, allowing it to easily get into cells of vaccinated people far more easily than other variants. But they suspect that Omicron has some other biological advantages as well. Last week, researchers reported that the variant carries a mutation that may weaken so-called innate immunity, a molecular alarm that rapidly activates our immune system at the first sign of an invasion in the nose. But it will take more experiments to see if this is indeed one of Omicron’s secrets to success. “It could be as simple as, this is a lot more virus in people’s saliva and nasal passages,” Dr. Cherry said. But there could be other explanations for its efficient spread: It could be more stable in the air, or better infect new hosts. “I think it’s really an important question,” she said.
|



 Your new post is loading...
Your new post is loading...