 Your new post is loading...

|
Scooped by
Juan Lama
|
Most people are still not eligible for a second booster, but the FDA will revisit the topic after an expert panel meets in June. The Food and Drug Administration on Tuesday approved a second omicron booster from Moderna and Pfizer for people over the age of 65 and immunocompromised individuals. Why it matters: An additional dose of the bivalent vaccine can help high-risk individuals with waning immunity to COVID, the FDA said. - Most people who have received a single shot of the bivalent vaccine aren't eligible for a second dose, but the FDA could revise criteria after an expert panel meets to discuss the topic in June.
Details: Tuesday's decision means those over 65 can get a second booster no sooner than four months after the first. - Immunocompromised people may receive the second shot at least two months after receiving the first dose "and additional doses may be administered at the discretion of, and at intervals determined by, their health care provider," the FDA said.
- The agency made additional changes, saying that unvaccinated people may receive a single dose of the bivalent vaccine as their first shot, rather than multiple doses of the original monovalent vaccine.
- Children between six months and five years old who have received up to three doses of the monovalent vaccine may receive a bivalent vaccine shot, but the number of doses they receive "will depend on the vaccine and their vaccination history," per the FDA.
- The monovalent shots from Moderna and Pfizer are no longer authorized for use in the U.S.
What they're saying: "At this stage of the pandemic, data support simplifying the use of the authorized mRNA bivalent COVID-19 vaccines and the agency believes that this approach will help encourage future vaccination," said Peter Marks, director of FDA's Center for Biologics and Research. - "Evidence is now available that most of the U.S. population 5 years of age and older has antibodies to SARS-CoV-2 ... either from vaccination or infection that can serve as a foundation for the protection provided by the bivalent vaccines," Marks added.
Catch up fast: An FDA advisory committee in January unanimously recommended that the U.S. replace initial doses of the original COVID monovalent shots with bivalent ones to target omicron subvariants, directing Pfizer, Moderna and Novavax to focus on bivalent vaccines. - At the time, the FDA requested that the panel consider a future immunization schedule and proposed a yearly one-dose schedule for the general population and two doses for high-risk individuals.
- The panel, however, did not make any specific recommendations on that because members said they needed additional data on different population groups to determine what dosage was appropriate for each group.
What we're watching: A panel for the Centers for Disease Control and Prevention is scheduled to meet on Wednesday to discuss the second booster strategy. - If panel recommends the shot for high-risk individuals and CDC Director Rochelle Walensky signs off, the boosters could be available this week.
Zoom out: The FDA's decision comes as a new subvariant, XBB. 1.16, known as Arcturus, has been spreading around the world and gaining traction in the U.S. Don't forget: The COVID public health emergency is set to end in less than a month, shifting the cost of vaccines and other countermeasures to the private market. FDA Press Release (April 18, 2023): https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-changes-simplify-use-bivalent-mrna-covid-19-vaccines

|
Scooped by
Juan Lama
|
The newly emerged SARS-CoV-2 Omicron BQ.1.1, XBB.1, and other sublineages have accumulated additional spike mutations that may affect vaccine effectiveness. Here we report neutralizing activities of three human serum panels collected from individuals 1-3 months after dose 4 of parental mRNA vaccine (post-dose-4), 1 month after a BA.5-bivalent-booster (BA.5-bivalent-booster), or 1 month after a BA.5-bivalent-booster with previous SARS-CoV-2 infection (BA.5-bivalent-booster-infection). Post-dose-4 sera neutralized USA-WA1/2020, BA.5, BF.7, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 SARS-CoV-2 with geometric mean titers (GMTs) of 1533, 95, 69, 62, 26, 22, and 15, respectively; BA.5-bivalent-booster sera improved the GMTs to 3620, 298, 305, 183, 98, 73, and 35; BA.5-bivalent-booster-infection sera further increased the GMTs to 5776, 1558,1223, 744, 367, 267, and 103. Thus, although BA.5-bivalent-booster elicits better neutralization than parental vaccine, it does not produce robust neutralization against the newly emerged Omicron BA.2.75.2, BQ.1.1, and XBB.1. Previous infection enhances the magnitude and breadth of BA.5-bivalent-booster-elicited neutralization. Preprint in bioRxiv (Nov. 02, 2022): https://doi.org/10.1101/2022.10.31.514580

|
Scooped by
Juan Lama
|
Background: Epidemiological evidence for immune imprinting was investigated in immune histories related to vaccination in Qatar from onset of the omicron wave, on December 19, 2021, through September 15, 2022. Methods: Matched, retrospective, cohort studies were conducted to investigate differences in incidence of SARS-CoV-2 reinfection in the national cohort of persons who had a primary omicron infection, but different vaccination histories. History of primary-series (two-dose) vaccination was compared to that of no vaccination, history of booster (three-dose) vaccination was compared to that of two-dose vaccination, and history of booster vaccination was compared to that of no vaccination. Associations were estimated using Cox proportional-hazards regression models. Results: The adjusted hazard ratio comparing incidence of reinfection in the two-dose cohort to that in the unvaccinated cohort was 0.43 (95% CI: 0.38-0.48). The adjusted hazard ratio comparing incidence of reinfection in the three-dose cohort to that in the two-dose cohort was 1.38 (95% CI: 1.16-1.65). The adjusted hazard ratio comparing incidence of reinfection in the three-dose cohort to that in the unvaccinated cohort was 0.53 (95% CI: 0.44-0.63). All adjusted hazard ratios appeared stable over 6 months of follow-up. Divergence in cumulative incidence curves in all comparisons increased markedly when incidence was dominated by BA.4/BA.5 and BA.2.75*. No reinfection in any cohort progressed to severe, critical, or fatal COVID-19. Conclusions: History of primary-series vaccination enhanced immune protection against omicron reinfection, but history of booster vaccination compromised protection against omicron reinfection. These findings do not undermine the short-term public health utility of booster vaccination. Preprint in medRxiv (Nov. 1, 2022): https://doi.org/10.1101/2022.10.31.22281756

|
Scooped by
Juan Lama
|
SARS-CoV-2 mRNA booster vaccines provide protection from severe disease, eliciting strong immunity that is further boosted by previous infection. However, it is unclear whether these immune responses are affected by the interval between infection and vaccination. Over a two-month period, we evaluated antibody and B-cell responses to a third dose mRNA vaccine in 66 individuals with different infection histories. Uninfected and post-boost but not previously infected individuals mounted robust ancestral and variant spike-binding and neutralizing antibodies, and memory B cells. Spike-specific B-cell responses from recent infection were elevated at pre-boost but comparatively less so at 60 days post-boost compared to uninfected individuals, and these differences were linked to baseline frequencies of CD27lo B cells. Day 60 to baseline ratio of BCR signaling measured by phosphorylation of Syk was inversely correlated to days between infection and vaccination. Thus, B-cell responses to booster vaccines are impeded by recent infection. Preprint available in medRxiv (August 31, 2022): https://doi.org/10.1101/2022.08.30.22279344

|
Scooped by
Juan Lama
|
A top F.D.A. regulator cited compelling data for redesigned coronavirus vaccines from Pfizer-BioNTech and Moderna. WASHINGTON — The Biden administration plans to offer the next generation of coronavirus booster shots to Americans 12 and older soon after Labor Day, a campaign that federal officials hope will reduce deaths from Covid-19 and protect against an expected winter surge. Dr. Peter Marks, the top vaccine regulator for the Food and Drug Administration, said in an interview on Tuesday that while he could not discuss timing, his team was close to authorizing updated doses that would target the versions of the virus now circulating. Even though those formulations have not been tested in humans, he said, the agency has “extremely good” data showing that the shots are safe and will be effective. “How confident am I?” he said. “I’m extremely confident.” This week, both Moderna and Pfizer-BioNTech finalized their submissions to the F.D.A. asking for emergency authorization of booster shots aimed at BA.5 and another subvariant of Omicron that together account for most coronavirus cases in the United States. Federal health officials say they are eager to offer the updated boosters as quickly as possible, pointing to a death toll that now averages about 450 Americans per day and could rise in the coming months as people spend more time indoors. “We have really got to do better to protect the American public,” Dr. Anthony S. Fauci, President Biden’s chief medical adviser, said in an interview on Tuesday. “We are in the middle of a BA.5 outbreak here, and we are nowhere near where we want to be.” The Biden administration has struggled to convince Americans of the need for successive vaccinations. Only about two-thirds of the population has been inoculated with the primary series of two shots, and far fewer have received booster doses. Some outside scientists have said the government is moving too fast to clear redesigned shots, arguing that the existing vaccines provide strong protection against severe disease. “Deaths are concentrated in unvaccinated people and people with serious health conditions,” said John P. Moore, a virologist at Weill Cornell Medicine. He said the extra protection that the new shots would provide against infection could be “weak to nonexistent.” Jeremy Kamil, a virologist at Louisiana State University Health Shreveport, said that although he supported new boosters, many people had immunity because of recent infections. “Even if we get this out in the next 10 days, how many people are left who haven’t gotten Omicron?” he said. Other scientists said that the government’s plan made sense given how the virus had changed and the evidence that immunity wanes over time. Dr. Marks said that if regulators waited for additional data or recommendations from outside experts, the virus might evolve further and “we may have lost a bunch of individuals who could otherwise be sitting around at the dinner table together.” In a sign of impending action from the F.D.A., the Centers for Disease Control and Prevention has scheduled a two-day meeting of its advisory panel of experts on the matter for Sept. 1 and 2. The C.D.C. director, Rochelle P. Walensky, would then make a final decision on whether to roll out the new doses. Shipments to states could begin as early as next week, according to officials familiar with the plan. The government plans to offer the new Pfizer booster to everyone 12 and older while limiting the new Moderna shot to adults. People who have already received the initial two-shot series of either vaccine would be eligible. So would those who have received the initial shots plus one or two booster shots. The new booster campaign could be broadened to younger children later. Dr. Marks suggested that the biggest obstacle to the effort was the level of complacency that had set in, even as the pandemic continued to exact what he called an “unacceptable” death toll. He said the F.D.A. might recommend that people who had recently received a Covid vaccine dose wait “a few months” before getting the new shot, even if they were otherwise eligible. He said the C.D.C. might weigh in on whether people who were recently infected with the virus should also wait. As of mid-August, the federal government had bought more than 170 million doses of the updated version of the vaccines. This month, the C.D.C. laid out detailed plans to offer the shots, warning that the supply would be “sufficient but finite” and saying that doses should be “directed to providers with expected demand among eligible patients.” The new shots combine the original vaccine with components aimed at the BA.4 and BA.5, Omicron’s recent subvariants. Officials argue that the new formulations will deliver a stronger boost to the immune system than the existing vaccines provide. Unlike earlier shots, the redesigned formulations have not been tested widely on humans; instead, the companies have submitted data from mice trials. Some vaccine experts have complained that animal data is too preliminary and say regulators should wait for results of human clinical trials. But Dr. Fauci said using animal data was “not anything different than we always do” in updating the flu vaccine each year. Dr. Marks said other evidence included the extensive track record with the existing vaccines and a series of earlier human trials with variant-specific formulations. “I take great issue with those who say, ‘Oh, you’re just approving this with mouse data,’” he said. “We’re authorizing this with the totality of the evidence that we have.” Moderna and Pfizer have both submitted clinical data from human studies of redesigned shots targeting the original version of the Omicron variant. Britain last week authorized that version of Moderna’s vaccine, but U.S. regulators asked for formulations aimed at Omicron’s newer subvariants. Researchers are still working to answer key questions about the protection that the new vaccines deliver, including the levels of antibodies the shots generate in humans and how those antibodies protect people. Moderna began human trials of its new vaccine this month, and Pfizer plans to do the same later in the month. Initial data from those trials is expected later this year. Dr. Moore, the virologist at Weill Cornell Medicine, said the administration’s plans could backfire if the fall or winter brings a wave of disease despite the new boosters, potentially reducing overall confidence in Covid-19 vaccines. “My issue all along has been: Is there enough data to really justify the effort?” Dr. Moore said. “The potential downside is, if the public thinks that this Omicron-containing booster is some kind of magic bullet that will give them superstrong protection from infection, is there a risk that they will change their behavior to increase their exposure?” The F.D.A. will decide whether to authorize the retooled doses without seeking a recommendation from its outside advisory panel of experts, a step it usually takes before making new vaccines available. Critics have complained that regulators have bypassed the panel at crucial steps. Dr. Marks defended the decision, saying a late June meeting of the advisory panel on the need to revise the vaccines had given regulators “everything we needed.” The committee voted overwhelming then in favor of updating the vaccines to work better against Omicron or its subvariants, but it did not consider specific formulations.

|
Scooped by
Juan Lama
|
Probably many people who watched or participated in the June 28 virtual US Food and Drug Administration (FDA) advisory committee meeting about updating COVID-19 vaccines could agree on 1 point, made by the agency’s Peter Marks, MD, PhD: “It is science at its hardest.” The FDA convened its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss whether to add an Omicron component to boosters for the fall. In order to have enough doses by early October, “we will need to very rapidly move to let companies know what that selection will be,” Marks reminded the panelists. (How many doses will be enough isn’t clear—as of June 30, only 51.1% of fully vaccinated US adults aged 18 years or older had received 1 booster shot, while only 27% of fully vaccinated adults aged 50 years or older, for whom a second booster is recommended, had received 2, according to government data.) Omicron, which the World Health Organization (WHO) classified as a variant of concern (VOC) in November 2021, is the first VOC that can evade the immune system, resulting in lower vaccine effectiveness, the WHO’s Kanta Subbarao, MBBS, MPH, told committee members. Even so, she noted, after a booster dose, the available prototype vaccines, which are based on the ancestral SARS-CoV-2 index virus strain that has long been undetectable among circulating viruses, continue to protect people against serious illness and death. After a day of listening to presentations by Subbarao, director of the WHO’s Collaborating Centre for Reference and Research on Influenza in Melbourne, and scientists from the FDA, the Centers for Disease Control and Prevention (CDC), and 3 vaccine manufacturers—Moderna, Pfizer, and Novavax—the advisory committee voted 19-2 to recommend inclusion of a SARS-CoV-2 Omicron component for COVID-19 booster vaccines this fall. Less than 48 hours later, the FDA, which usually follows advisory committee recommendations after such lopsided votes, announced that manufacturers seeking to update COVID-19 vaccines should add a spike protein component of the Omicron subvariants BA.4 and BA.5 (which differ only outside the spike protein) to their prototype vaccines to make bivalent boosters that can be used beginning this fall. “[W]e have not advised manufacturers to change the vaccine for primary vaccination, since a primary series with the FDA-authorized and approved COVID-19 vaccines provide a base of protection against serious outcomes of COVID-19 caused by circulating strains of SARS-CoV-2,” Marks, director of the agency’s Center for Biologics Evaluation and Research, said in the announcement. Yet the data presented at the VRBPAC meeting were about experimental bivalent boosters that combined prototype vaccines with the spike protein of the original Omicron variant, BA.1. When the advisory committee reconvened after lunch, Marks noted that in the week ending June 25, BA.4 and BA.5 represented more than half of US circulating SARS-CoV-2 variants for the first time, according to newly released CDC data. By the week ending July 2, BA.5 alone represented 53.6% of US circulating SARS-CoV-2 variants, while BA.4 represented 16.5%, according to the CDC. CDC data show that BA.1, which represented about a third of circulating US variants on March 26, was undetectable by May 21—an illustration of just how quickly SARS-CoV-2 is evolving.....

|
Scooped by
Juan Lama
|
Background The duration of protection against the omicron (B.1.1.529) variant for current COVID-19 vaccines is not well characterised. Vaccine-specific estimates are especially needed. We aimed to evaluate the effectiveness and durability of two and three doses of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine against hospital and emergency department admissions due to the delta (B.1.617.2) and omicron variants. Methods In this case–control study with a test-negative design, we analysed electronic health records of members of Kaiser Permanente Southern California (KPSC), a large integrated health system in California, USA, from Dec 1, 2021, to Feb 6, 2022. Vaccine effectiveness was calculated in KPSC patients aged 18 years and older admitted to hospital or an emergency department (without a subsequent hospital admission) with a diagnosis of acute respiratory infection and tested for SARS-CoV-2 via PCR. Adjusted vaccine effectiveness was estimated with odds ratios from adjusted logistic regression models. This study is registered with ClinicalTrials.gov (NCT04848584). Findings Analyses were done for 11 123 hospital or emergency department admissions. In adjusted analyses, effectiveness of two doses of the BNT162b2 vaccine against the omicron variant was 41% (95% CI 21–55) against hospital admission and 31% (16–43) against emergency department admission at 9 months or longer after the second dose. After three doses, effectiveness of BNT162b2 against hospital admission due to the omicron variant was 85% (95% CI 80–89) at less than 3 months but fell to 55% (28–71) at 3 months or longer, although confidence intervals were wide for the latter estimate. Against emergency department admission, the effectiveness of three doses of BNT162b2 against the omicron variant was 77% (72–81) at less than 3 months but fell to 53% (36–66) at 3 months or longer. Trends in waning against SARS-CoV-2 outcomes due to the delta variant were generally similar, but with higher effectiveness estimates at each timepoint than those seen for the omicron variant. Interpretation Three doses of BNT162b2 conferred high protection against hospital and emergency department admission due to both the delta and omicron variants in the first 3 months after vaccination. However, 3 months after receipt of a third dose, waning was apparent against SARS-CoV-2 outcomes due to the omicron variant, including hospital admission. Additional doses of current, adapted, or novel COVD-19 vaccines might be needed to maintain high levels of protection against subsequent waves of SARS-CoV-2 caused by the omicron variant or future variants with similar escape potential. Published (April 22, 2022) in The Lancet Respiratory Medicine:

|
Scooped by
Juan Lama
|
Background. The purpose of this study was to evaluate whether boosting healthcare personnel, already reasonably protected by prior infection or vaccination, with a vaccine developed for an earlier variant of COVID-19 protects against the Omicron variant. Methods. Employees of Cleveland Clinic who were previously infected with or vaccinated against COVID-19, and were working in Ohio the day the Omicron variant was declared a variant of concern, were included. The cumulative incidence of COVID-19 was examined over two months during an Omicron variant surge. Protection provided by boosting (analyzed as a time-dependent covariate) was evaluated using Cox proportional hazards regression. Analyses were adjusted for time since proximate overt immunologic challenge (POIC) as a time-dependent covariate. Results. Among 39 766 employees, 8037 (20%) previously infected and the remaining previously vaccinated, COVID-19 occurred in 6230 (16%) during the study. Risk of COVID-19 increased with time since POIC. In multivariable analysis, boosting was independently associated with lower risk of COVID-19 among those with vaccine-induced immunity (HR, .43; 95% CI, .41-.46) as well as those with natural immunity (HR, .66; 95% CI, .58-.76). Among those with natural immunity, receiving 2 compared to 1 dose of vaccine was associated with higher risk of COVID-19 (HR, 1.54; 95% CI, 1.21-1.97). Conclusions. Administering a COVID-19 vaccine not designed for the Omicron variant, 6 months or more after prior infection or vaccination, protects against Omicron variant infection in both previously infected and previously vaccinated individuals. There is no evidence of an advantage to administering more than 1 dose of vaccine to previously infected persons. Preprint available at medRxiv (Feb. 13, 2022): https://doi.org/10.1101/2022.02.10.22270744

|
Scooped by
Juan Lama
|
There are concerns that the SARS-CoV-2 Omicron variant evades immune responses due to unusually high numbers of mutations on the spike protein. Here we report a super-spreading event of Omicron infections amongst triple-vaccinated healthcare workers, infecting 21 of 33 attending a private gathering in the Faroe Islands. The incubation period of Omicron was short in this study. If the incubation period for Omicron is shorter than for previous variants, this can potentially partly explain the increased infection in individuals with some immunity. It is not possible to determine hospitalization rate or death rates from this small study. We do not yet know the risk of developing Long Covid after an Omicron infection. Even if the cases in this study primarily experienced relatively mild disease, all the reported cases have had previous immunity through vaccination. It is notable, that all the infected cases experienced symptoms, and that especially loss of taste and smell seem to be less common in these cases, compared with previous outbreaks. It is likely that vaccination also protects against severe disease with the Omicron variant, even if protection against infection has waned to some degree, still underlining the importance of vaccination. Of note, the findings might not generalize to SARS-CoV-2 naive individuals, and for this reason, further research in Omicron amongst SARS-CoV-2 naive individuals is needed. Preprint available at medRxiv (Dec. 23, 2021): https://doi.org/10.1101/2021.12.22.21268021

|
Scooped by
Juan Lama
|
BACKGROUND The emergence of the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 and the reduced effectiveness over time of the BNT162b2 vaccine (Pfizer–BioNTech) led to a resurgence of coronavirus disease 2019 (Covid-19) cases in populations that had been vaccinated early. On July 30, 2021, the Israeli Ministry of Health approved the use of a third dose of BNT162b2 (booster) to cope with this resurgence. Evidence regarding the effectiveness of the booster in lowering mortality due to Covid-19 is still needed. METHODS We obtained data for all members of Clalit Health Services who were 50 years of age or older at the start of the study and had received two doses of BNT162b2 at least 5 months earlier. The mortality due to Covid-19 among participants who received the booster during the study period (booster group) was compared with that among participants who did not receive the booster (nonbooster group). A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of booster status with death due to Covid-19, with adjustment for sociodemographic factors and coexisting conditions. RESULTS A total of 843,208 participants met the eligibility criteria, of whom 758,118 (90%) received the booster during the 54-day study period. Death due to Covid-19 occurred in 65 participants in the booster group (0.16 per 100,000 persons per day) and in 137 participants in the nonbooster group (2.98 per 100,000 persons per day). The adjusted hazard ratio for death due to Covid-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% confidence interval, 0.07 to 0.14; P<0.001). CONCLUSIONS Participants who received a booster at least 5 months after a second dose of BNT162b2 had 90% lower mortality due to Covid-19 than participants who did not receive a booster. Published in NEJM (Dec. 8, 2021): https://doi.org/10.1056/NEJMoa2115624

|
Scooped by
Juan Lama
|
If the C.D.C. agrees, adults who received a second shot of the Pfizer or Moderna vaccine at least six months ago could be eligible by this weekend. WASHINGTON — The Food and Drug Administration on Friday authorized booster shots of both the Pfizer-BioNTech and Moderna vaccines for everyone 18 and older, opening up eligibility to tens of millions more fully vaccinated adults. The move simplifies eligibility, fulfills a pledge by President Biden to offer the shots to every American adult and formally allows a practice already in place in at least 10 states. Fearful that waning protection and the onset of winter will set off a wave of breakthrough infections, a growing number of governors had already offered boosters to everyone 18 and older ahead of the holidays. The agency said the expansion was justified by currently available clinical trial data as well as real-world evidence. In a statement, Dr. Peter Marks, who leads the F.D.A. division that regulates vaccines, added: “Streamlining the eligibility criteria and making booster doses available to all individuals 18 years of age and older will also help to eliminate confusion about who may receive a booster dose and ensure booster doses are available to all who may need one.” Dr. Anthony S. Fauci, the federal government’s top infectious disease expert, has argued relentlessly over the past month for booster shots for all adults, a position shared by most of Mr. Biden’s other health advisers. Public health experts who argue that healthy younger adults do not need them, he has said, are ignoring the risks of symptomatic Covid-19. “Enough is enough. Let’s get moving on here,” he said at an event Wednesday night. “We know what the data are.” If the Centers for Disease Control and Prevention agrees, all adults who received a second shot of either Pfizer or Moderna at least six months ago will most likely be able to get a booster shot by the weekend. A meeting of the agency’s outside advisers is scheduled for Friday. At a White House briefing on Wednesday, Dr. Rochelle Walensky, the C.D.C. director, promised that the agency would “quickly review the safety and effectiveness data and make recommendations as soon as we hear from F.D.A.” The F.D.A.’s action came after months of fierce debate within the administration and the scientific community about who needed booster shots, and when. As large studies showed protection from all three federally authorized vaccines holding strongly, some outside advisers to the F.D.A. and C.D.C. repeatedly expressed discomfort with how quickly the administration was moving to offer the shots. While the F.D.A. has been evaluating booster data for months, it granted the companies’ requests with remarkable speed. Pfizer-BioNTech filed its request roughly 10 days ago, and Moderna announced Wednesday that it had applied but did not say when. The agency said that it did not convene its independent vaccine advisory committee because the panel had already had discussed the use of booster shots extensively and “the requests do not raise questions that would benefit from additional discussion.” The panel’s recommendations are nonbinding. For Moderna, regulators said, the data showed that 149 clinical trial participants who received a booster dose had higher antibody levels compared with 1,055 study volunteers who received only two doses. They said Pfizer’s data for 200 participants in a clinical trial showed the booster shot raised antibody levels compared to one month after a second shot. Critics have said the administration’s booster campaign was motivated by practical or political reasons more than scientific ones. Some said that federal regulators were analyzing safety and efficacy data on the fly. Others have worried that moving to clear Pfizer and Moderna booster doses for all adults might inadvertently undermine the vaccines and suggest that two doses are not strongly protective. “The evidence isn’t there that a large rollout of boosters is really going to have that much impact on the epidemic,” said Ira M. Longini Jr., a vaccine expert and professor of biostatistics at the University of Florida. He said that booster doses could increase protection at least temporarily and might help a jittery public feel as if they have another tool in the pandemic, but would do little to halt transmission of the virus, which is being driven by the unvaccinated. He repeated the administration’s own oft-stated position that persuading the unvaccinated to get the shots must remain the top priority. Other public health experts have argued that the government needed to offer boosters to all adults to eliminate confusion. Complex eligibility rules coupled with the government’s recent decision to let people choose among all three vaccines for their booster has left the public somewhat befuddled, they say. “This decision by F.D.A. is overdue,” said Dr. Elizabeth McNally, director of the Center for Genetic Medicine at Northwestern University’s Feinberg School of Medicine. “Many people had trouble understanding whether they should or shouldn’t get boosters,” she said in a statement. “This message is much clearer — get a booster!”.... FDA release (Nov. 19, 2021) available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters

|
Scooped by
Juan Lama
|
Vaccine advisers to the US Food and Drug Administration voted unanimously Friday to recommend a booster dose of Johnson & Johnson's vaccine at least two months after people get the first dose. The FDA's Vaccines and Related Biological Products Advisory Committee voted 19-0 to recommend the extra dose for all recipients of the J&J Janssen vaccine, 18 and older. They asked to simplify the original question being posed by the FDA, which had asked the committee to say whether the data showed that waiting six months or longer after getting the first shot would provide an even stronger immune response. The FDA will now consider the committee's advice. Then the US Centers for Disease Control and Prevention's vaccine advisers will be asked to consider it. Johnson & Johnson says studies have shown boosting at two or six months can bring that effectiveness up to 94% and it says its effectiveness does not wane over time in the same way that effectiveness from Pfizer's vaccine does. But the Johnson & Johnson vaccine has not been shown to be as protective as either the Pfizer or Moderna vaccines, noted VRBPAC chair Dr. Arnold Monto, a professor of public health and epidemiology at the University of Michigan. "So there is some urgency here to do something," he told the meeting. And the CDC's Dr. Amanda Cohn told the meeting that various studies suggested real-world efficacy of J&J's vaccine was anywhere between 50% and 68%. "Regardless of whether or not there been waning or this is the true effectiveness after a single dose, the effectiveness or protection of a single dose of the J&J vaccine is not equivalent to protection at this time with either two doses of an mRNA vaccine and certainly not in those groups who have now been authorized to receive a booster dose of an mRNA vaccine," Cohn said. Members of the committee said while there was not much data to show whether the efficacy of the Janssen vaccine was waning -- or was strong to begin with -- they agreed people should be given the opportunity for a booster. "I would say I agree a second dose booster is needed to boost immunity back to the 90-plus range," Dr. Archana Chatterjee, a pediatric infectious diseases expert at Rosalind Franklin University in Chicago, said before the vote. The FDA has already given EUA to a booster for Pfizer's vaccine for people who are six months out from their first two shots who are also either 65 or older or who are at least 18 and have a higher risk of severe disease because of pre-existing conditions or because of work or living conditions. And Americans are already flocking to get those boosters. Data from the US Centers for Disease Control and Prevention show close to 5% of fully vaccinated people -- about 9 million people -- have received booster shots. On Thursday, VRBPAC members voted unanimously to recommend booster doses of Moderna's vaccine to the same groups. If the FDA gives emergency use authorization to Moderna or Johnson & Johnson boosters, CDC vaccine advisers will meet to discuss which groups to recommend them to. Typically, shots can be administered once the CDC director signs off on the recommendation. CDC's Advisory Committee on Immunization Practices is scheduled to discuss boosters on October 21.

|
Scooped by
Juan Lama
|
Those eligible for the extra shot would include adults over 65 and others at high risk — the same groups now eligible for a Pfizer-BioNTech boost. WASHINGTON — A panel of independent medical experts on Thursday unanimously recommended Moderna booster shots for many of those who had received the company’s coronavirus vaccine, paving the way to sharply expand the number of people eligible for an additional shot in the United States. The advisory panel to the Food and Drug Administration voted 19 to 0 in favor of emergency authorization of a half-dose booster, at least six months after the second dose. Those eligible for the extra shot would include people over 65 and other adults considered at high risk — the same groups now eligible for a Pfizer-BioNTech booster. The F.D.A. typically follows the panel’s advice, and should rule within days. The recommendations come as the nation is seeing a decline in coronavirus cases but still faces nearly 90,000 new infections and roughly 2,000 deaths per day. The Biden administration has cast booster shots as an additional tool in the battle against the pandemic, while acknowledging that controlling the disease’s spread depends upon vaccinating tens of millions of Americans for the first time. In a speech at the White House on Thursday, President Biden once again sought to rally businesses to support vaccination mandates that he said would help reduce the ranks of the unvaccinated in the United States, calling the number of people who have not gotten even a first shot “unacceptably high.” Mr. Biden encouraged Americans to seek out booster shots when they become eligible, calling them “free, available and convenient.” More than seven million people in the United States have already obtained booster doses of the Pfizer-BioNTech vaccine, and more than a million have received third doses of Moderna’s, even though only Moderna recipients with immune deficiencies are officially eligible. Thursday’s vote was considerably smoother than the one the panel held last month, after a chaotic and at times acrimonious debate on whether the F.D.A. should authorize booster shots for Pfizer-BioNTech recipients. On Friday, the same expert committee will meet to discuss and vote on whether the roughly 15 million people who received the single-dose Johnson & Johnson vaccine should also be eligible for booster doses. Its members are also supposed to discuss a new federal study that suggeststhose Americans might be better off getting a booster dose of the Moderna or Pfizer-BioNTech vaccines. If the F.D.A. quickly authorizes Moderna booster doses, and if the Centers for Disease Control and Prevention signs off after a meeting of its own committee of vaccine experts next week, people in the eligible groups could begin seeking out the shots soon afterward. In addition to people over 65, younger adults at high risk of severe Covid-19 or serious complications because of pre-existing medical conditions or exposure at work would also become eligible. Some committee members on Thursday decried the lack of more robust data justifying a booster. Dr. Archana Chatterjee, an infectious disease expert at Rosalind Franklin University, pointed out that cases were already declining here without widely available booster doses. Other members said that the F.D.A. had set a precedent by authorizing additional shots for many recipients of the Pfizer-BioNTech vaccine, making it hard to deny vulnerable Americans who had received the Moderna vaccine a chance to receive a booster. “From a pragmatic point of view,” said Dr. Stanley Perlman of the University of Iowa, a committee member, “because we’ve already approved it for Pfizer, I don’t see how we can possibly not approve it for Moderna.” To date, more than 103 million people in the United States have been fully vaccinated with Pfizer’s product, more than 69 million with Moderna’s and about 15 million with the Johnson & Johnson shot. Dr. Patrick Moore, a panel member and virologist at the University of Pittsburgh, said he voted to recommend a Moderna booster based on a “gut feeling, rather than based on really, truly serious data.” Others questioned whether half a dose was the right amount, and whether a Moderna booster would work better if it was given at least eight months after the second shot, instead of six months. “I’m not sure we have actually identified the optimal regimen for these vaccines,” said Dr. Michael Kurilla, an infectious disease expert at the National Institutes of Health. The panel made clear it did not favor expanding eligibility for booster shots beyond the higher-risk groups that qualify for Pfizer boosters. No vote was taken on that question, but the committee expressed concern that booster eligibility decisions could become a slippery slope. Several experts said they were worried about recommending additional shots based on clinical trial results from just a few hundred participants. “I’m not sure that we want to just explore it willy-nilly by giving it to a lot of people,” said Dr. Eric Rubin, an adjunct professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health. Dr. Peter Marks, the F.D.A.’s top vaccine regulator, said the agency’s experts would take the panel’s concerns to heart in considering whether younger adults without significant risk factors should become eligible for boosters. The agency “heard pretty loud and clearly that there was not a lot of appetite for moving down the age range very significantly, if at all,” he said. State health officials say that the staggered rollout of boosters has left some especially vulnerable people — for example, elderly residents of nursing homes who got the Moderna vaccine — in the lurch. But some panel members said on Thursday that nearly two months after the Biden administration announced its booster plan, the rationale remained vague.......
|

|
Scooped by
Juan Lama
|
The Biden administration is moving to make the shot available for older people and those with impaired immune systems. While federal authorities are poised to authorize a second dose of the omicron-targeting coronavirus booster for vulnerable groups, not all experts believe it is necessary. (Kristopher Radder/Brattleboro Reformer/AP) Federal regulators have decided to authorize a second omicron-specific coronavirus vaccine booster shot for people who are at least 65 or have weak immune systems — an effort to provide additional protection to high-risk individuals, according to several officials familiar with the plan. The Food and Drug Administration is expected to announce the step in the next few weeks, and the Centers for Disease Control and Prevention is expected to move quickly to endorse it, said the officials, who spoke on the condition of anonymity because they were not authorized to publicly discuss internal discussions. Eligible individuals will be able to receive the dose as long as it has been at least four months since their first shot of what’s known as the bivalent booster, which targets omicron subvariants BA.4 and BA.5 as well as the original novel coronavirus. The expectation is that consumers will consult with their health-care providers about whether to get the extra booster, the officials said. The FDA’s policy change will be “permissive” — people may get the shot but will not be told they should get it, the officials said. It’s not clear whether the CDC’s vaccine advisory committee would meet to discuss the change before CDC Director Rochelle Walensky makes a final recommendation. Doctors and other experts have expressed mixed views about a second bivalent booster. Some say there is little data to justify it, while others argue it would benefit high-risk individuals who received their first omicron-targeting shot last fall and probably have reduced protection as the effects fade. Some anxious patients have been “really clamoring” for a second omicron booster, said Camille Kotton, clinical director for transplant and immunocompromised host infectious diseases at Massachusetts General Hospital in Boston. At a recent meeting of the CDC vaccine advisory panel, she said she would support allowing additional booster doses for high-risk groups, especially for the most significantly immunocompromised patients. Jamie Loehr, a family medicine doctor in Ithaca, N.Y., who is also a member of the vaccine advisory panel, said there is evidence that older people and those with weak immune systems don’t produce especially robust responses to vaccines in general, and to the coronavirus vaccine in particular. It would seem reasonable to give them more frequent boosters, he wrote in an email, but he wants to see data before deciding whether he would support a more frequent booster for these groups. John P. Moore, a professor of microbiology and immunology at Weill Cornell Medicine in New York, said an extra booster could benefit people who are in poor health or have an impaired immune system. But he was skeptical everyone older than 65 needs it. Boosters lead to “a short-term boost against mild infection but protection against severe disease is still pretty robust” because of previous shots, he said. Moore said it is a mistake to think “everyone over a certain age is in the same health bracket,” when in fact health status varies widely. He said he is older than 65 and healthy — and “not giving a moment’s thought about getting another booster, though I might next winter if infections tick up.” Administration officials acknowledge there is not extensive data on the bivalent vaccine, which was first authorized in August. But they said real-world data and smaller studies are consistent with large studies on the original vaccine showing that its protection against symptomatic infection fades after several months. In addition, unpublished data presented at the CDC’s vaccine advisory panel meeting in February confirmed earlier real-world reports that bivalent vaccines are providing protection against serious illness — emergency room visits and hospitalizations — in adults, compared with people who received previous doses of the original vaccine and no omicron-targeting dose. Other studies also suggest older people might be better shielded from serious illness with an additional booster, the officials said. They noted they are not advocating the second omicron booster for young people, who might experience rare heart-related side effects. The Wall Street Journal and NPR previously reported that the FDA was considering a second bivalent booster for high-risk groups. Only about 42 percent of people 65 and older have received the first bivalent booster dose, according to the CDC. A second booster will not affect the FDA’s plan to move to a once-a-year coronavirus vaccine booster for most Americans — a strategy announcedby the agency in January, the officials said. This summer, the agency and its advisers will select a retooled formula for an updated vaccine to be deployed during a campaign planned for next fall. The formula will be based on which coronavirus strain scientists think will be circulating in the fall and winter. In a statement, the FDA said: “We continue to closely monitor the emerging data in the United States and globally, and we will base any decision on additional updated boosters upon those data. Importantly, individuals who have not yet received an updated (bivalent) booster are encouraged to speak with their health-care provider and consider receiving one.” The updated shot will be free, regardless of insurance coverage, because the government has an ample supply of boosters. Even after the federal supply of vaccines is gone, shots will continue to be free to most people with private and public health insurance. But once federally purchased doses are depleted, uninsured and underinsured adults may have to pay, and privately insured people might need to confirm that their provider is in-network, according to an analysis by the Kaiser Family Foundation. Peter Hotez, dean of the National School of Tropical Medicine and professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine in Houston, said he is a strong proponent of making a second omicron-targeting booster available for everyone 50 and older. Michael T. Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, agreed, saying a second booster would provide an additional tool for people who have been conscientious about reducing their risks of contracting covid. An omicron subvariant, XBB.1.5, now accounts for almost 90 percent of the cases in the United States, according to the CDC.

|
Scooped by
Juan Lama
|
Neutralizing antibodies that target the BA.4 and BA.5 subvariants were four-fold higher in people aged 55 and older who received the bivalent booster than in those who received a monovalent booster. New data from Pfizer and BioNTech on their bivalent Covid-19 vaccine suggests the updated product may be more protective against more recent Omicron subvariants than the original version of the vaccine, the companies said in a statement released Friday. The companies said the levels of neutralizing antibodies that target the BA.4 and BA.5 subvariants of the SARS-CoV-2 virus were four-fold higher in people aged 55 and older who received the bivalent booster than in similarly aged people who received a monovalent booster. The bivalent, which was given an emergency use authorization at the end of August, targets both the original version of the SARS-2 virus and the BA.4/BA.5 variants. Recently BA.5 has been the dominant strain in the United States, but an alphabet soup of newer subvariants — BA.4.6, BQ.1.1 among them — is starting to crowd it out. The new data from the companies only looks at what getting the the booster did to antibody levels in recipients. The trial did not test whether people who received the updated boosters were less likely to contract Covid than people who received one of the older boosters. “These data demonstrate that our BA.4/BA.5-adapted bivalent vaccine works as conceptually planned in providing stronger protection against the Omicron BA.4 and BA.5 sublineages,” Ugur Sahin, CEO and co-founder of BioNTech, said in the statement. “In the next step and as part of our science-based approach, we will continue to evaluate the cross-neutralization of the adapted vaccine against new variants and sublineages. Our aim is to provide broader immunity against Covid-19 caused by SARS-CoV-2, including Omicron and other circulating strains.” The companies also reported that one month after the trial participants got a dose of the bivalent booster, neutralizing antibodies targeting Omicron BA.4/BA.5 viruses increased 13.2-fold from pre-booster levels in adults who were older than 55 years of age; they increased 9.5-fold for adults 18 to 55 years of age. By comparison, in adults older than 55 who received a booster dose of the original vaccine, antibody titers to BA.4 and BA.5 rose 2.9-fold over the same period. Of late there have been a number of small studies that have tried to get an answer to the question of whether the updated vaccines are likely to be more protective than the original version against Omicron viruses. Three concluded that updating the vaccine did not make a difference while two suggested there was a benefit. But differences in the designs of the studies make them hard to compare to each other and to the Pfizer data. And at the end of the day, the important question is whether what was seen in terms of antibody production will translate into better protection for people who receive the bivalent vaccine, said Florian Krammer, a vaccinologist at Mount Sinai School of Medicine in Manhattan. He thought it might.
“A four-fold higher titer, that would be good,” said Krammer, who has done some paid consulting work for Pfizer. “Four-fold is usually the magical cut-off for a lot of us when we look at neutralization. Four-fold seems to mean something.” He cautioned though, that with different groups coming up with different estimates of whether the bivalent vaccine generated a significant improvement in antibody levels, “we really need to figure out what the truth is here and who is right in terms of measuring.” Eric Topol, director of the Scripps Research Translational Institute, thought the results were promising. “I think this is encouraging,” Topol told STAT. “We just need more people to get the darn booster.” Data from the Centers for Disease Control show uptake of the bivalent boosters has been slow, with only 26.3 million people having received it so far. Even in the highest risk age group, people 65 and older, uptake has been modest. To date only 23% of Americans in that age group have received a bivalent booster. Pfizer press release (Nov. 04, 2022) available here: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-updated-clinical-data-omicron

|
Scooped by
Juan Lama
|
BACKGROUND The safety and immunogenicity of the bivalent omicron-containing mRNA-1273.214 booster vaccine are not known. METHODS In this ongoing, phase 2–3 study, we compared the 50-μg bivalent vaccine mRNA-1273.214 (25 μg each of ancestral Wuhan-Hu-1 and omicron B.1.1.529 [BA.1] spike messenger RNAs) with the previously authorized 50-μg mRNA-1273 booster. We administered mRNA-1273.214 or mRNA-1273 as a second booster in adults who had previously received a two-dose (100-μg) primary series and first booster (50-μg) dose of mRNA-1273 (≥3 months earlier). The primary objectives were to assess the safety, reactogenicity, and immunogenicity of mRNA-1273.214 at 28 days after the booster dose. RESULTS Interim results are presented. Sequential groups of participants received 50 μg of mRNA-1273.214 (437 participants) or mRNA-1273 (377 participants) as a second booster dose. The median time between the first and second boosters was similar for mRNA-1273.214 (136 days) and mRNA-1273 (134 days). In participants with no previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the geometric mean titers of neutralizing antibodies against the omicron BA.1 variant were 2372.4 (95% confidence interval [CI], 2070.6 to 2718.2) after receipt of the mRNA-1273.214 booster and 1473.5 (95% CI, 1270.8 to 1708.4) after receipt of the mRNA-1273 booster. In addition, 50-μg mRNA-1273.214 and 50-μg mRNA-1273 elicited geometric mean titers of 727.4 (95% CI, 632.8 to 836.1) and 492.1 (95% CI, 431.1 to 561.9), respectively, against omicron BA.4 and BA.5 (BA.4/5), and the mRNA-1273.214 booster also elicited higher binding antibody responses against multiple other variants (alpha, beta, gamma, and delta) than the mRNA-1273 booster. Safety and reactogenicity were similar with the two booster vaccines. Vaccine effectiveness was not assessed in this study; in an exploratory analysis, SARS-CoV-2 infection occurred in 11 participants after the mRNA-1273.214 booster and in 9 participants after the mRNA-1273 booster. CONCLUSIONS The bivalent omicron-containing vaccine mRNA-1273.214 elicited neutralizing antibody responses against omicron that were superior to those with mRNA-1273, without evident safety concerns. (Funded by Moderna; ClinicalTrials.gov number, NCT04927065. opens in new tab.) Published in NEJM (Sept.16, 2022): https://doi.org/10.1056/NEJMoa2208343

|
Scooped by
Juan Lama
|
WASHINGTON — The Food and Drug Administration on Wednesday authorized the first redesign of coronavirus vaccines since they were rolled out in late 2020, setting up millions of Americans to receive new booster doses targeting Omicron subvariants as soon as next week. The agency cleared two options aimed at the BA.5 variant of Omicron that is now dominant: one made by Pfizer and its German partner BioNTech for use in people as young as 12, and the other by Moderna, for those 18 and older. The doses can be given at least two months since people last received a booster dose or completed their initial series of vaccinations. Biden administration officials have argued that even as researchers work to understand how protective the new shots might be, inoculating Americans again in the coming weeks could help curb the persistently high number of infections and deaths. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose,” Dr. Robert M. Califf, the F.D.A. commissioner, said in a statement on Wednesday. He added that the vaccine would “provide better protection against currently circulating variants.” An average of about 90,000 infections and 475 deaths are recorded every day around the United States, almost three years into a pandemic that has killed more than a million Americans and driven a historic drop in life expectancy. But there are also hopeful signs. Even with high case counts, fewer than 40,000 people are currently hospitalized with the virus, a decrease of 10 percent since early August and far fewer than during the Delta-driven surge last summer or the Omicron-fueled wave last winter. Deaths have also remained somewhat flat in recent weeks, a sign that vaccines are helping to prevent the worst outcomes of Covid-19. Ample evidence suggests that many Americans will hold back from getting the updated boosters, either because they are weary of the pandemic or may not feel urgency about an additional dose. With each new shot offered, there are fewer takers. As more companies bring workers back to offices and students return to campuses this fall, persuading Americans to get the updated booster shots will be a major challenge for the administration. The companies produced the retooled shots with extraordinary speed, a testament to the mRNA technology that Pfizer and Moderna have harnessed since the early months of the coronavirus outbreak. The Food and Drug Administration advised companies only two months ago on the formulation that they should adopt for the new vaccines. By later this week, millions of those doses will be delivered to states. The tight timeline meant that the companies went to federal regulators this summer with more limited data on the redesigned boosters than a traditional review process would call for, generating some controversy. Regulators authorized the vaccine without results from human trials, which have just started. Federal officials argue that because the coronavirus is evolving so quickly, human trials would be out of date before they can deliver results that could be used in the F.D.A.’s authorization decision. Instead, they are relying on the results of mouse trials and earlier human trials by Pfizer and Moderna of reformulations aimed at previous versions of the virus. The Biden administration is casting the shots as a standard upgrade that Americans should embrace ahead of potential surges in cases in the winter, like the flu shot, which is reconfigured every year to target more current versions of the influenza virus. The boosters are arriving during a period when the White House has been largely quiet on the pandemic. President Biden has rarely commented on the coronavirus in recent months, even after he tested positive in July. The White House no longer holds regular news briefings on the federal pandemic response, as it did in the first year of the administration — a reflection of the weariness of many Americans in keeping up with Covid precautions. “Covid-19 is the third leading cause of death in the United States. And it’s as if we’ve just accepted that that is going to be the case,” said Mercedes Carnethon, an epidemiologist at Northwestern University’s Feinberg School of Medicine. “I really hope as many people as possible will seek the updated booster so we can protect those who will have a terrible outcome.” Vaccinations remain the cornerstone of the federal government’s Covid strategy, even with tests and treatments widely available. The Biden administration has ordered over 170 million doses for the fall campaign, and officials do not expect shortages when they are rolled out. “If it’s freezing cold out and you have children, you’re going to dress them warmly. This is the concept here,” said Dr. Paul G. Auwaerter, the clinical director of the infectious diseases division at the Johns Hopkins University School of Medicine. “You’ll want to head into the respiratory season with a virus that we know has surprised us with a booster.” Exactly how protective the shots might be is unknown, Dr. Auwaerter said. He pointed to the modest increases in neutralizing antibodies that the companies found in vaccines they tested this year that targeted the original form of Omicron. How antibody levels would translate to protection with the new vaccines was unclear, he added. Experts warned against trying to choose Moderna’s shot over Pfizer’s or vice versa; with research in humans just beginning, scientists are months from knowing whether one brand offers better protection than the other. Many Americans have recently been infected with variants in the Omicron family and have some protection from their bouts with the virus, a development that federal agencies may take into account when recommending how the new shots are used. An advisory committee to the Centers for Disease Control and Prevention is scheduled to meet this week to make recommendations. “For most people, the risk of death is so low at this point, because they’ve gotten infected or vaccinated, or more likely both,” said Dr. Gregory A. Poland, a professor of medicine and infectious diseases and the director of the Vaccine Research Group at the Mayo Clinic. Dr. Poland, who has advised Moderna, Pfizer and White House officials on coronavirus vaccines, said updating booster shots the way the Food and Drug Administration did on Wednesday amounted to a “chase your tail” strategy, tweaking the design incrementally to try to keep up with a fast-changing virus. The new boosters, he said, could potentially save some lives among the elderly and those with immune deficiencies. But they were unlikely to make as substantial an impact with the rest of the population.

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration is using a controversial strategy to evaluate the next generation of COVID-19 boosters. The approach is stirring debate as the agency works to make new, hopefully improved, boosters available in September to help prevent severe disease and save lives in the fall and winter. For the first time, the FDA is planning to base its decision about whether to authorize new boosters on studies involving mice instead of humans. "For the FDA to rely on mouse data is just bizarre, in my opinion," says John Moore, an immunologist at Weill Cornell Medicine in New York. "Mouse data are not going to be predictive in any way of what you would see in humans." But others defend the approach, arguing that the country has had enough experience with the vaccines at this point to be confident the shots are safe and that there's not enough time to wait for data from human studies. "We have 500 people a day dying of coronavirus right now. Those numbers sadly might very well rise in the fall and the winter. The question is: 'Can we do something better?'" says Dr. Ofer Levy, a pediatrics and infectious disease researcher at Harvard Medical School who also advises the FDA. "And I think the answer is: 'We can, by implementing this approach.'" The U.K. just approved a new booster The United Kingdom just approved a new booster that targets both the original strain of the virus and the original omicron variant, called BA.1 — a so-called bivalent vaccine. But the FDA rejected BA.1 bivalent boosters last spring. Instead, the FDA told the vaccine companies that make the mRNA vaccines, Moderna and Pfizer and BioNTech, to develop bivalent vaccines that target the dominant omicron subvariants — BA.4 and BA.5 — in the hopes they will offer stronger, longer-lasting protection. That's why the FDA decided to use a new, streamlined strategy for testing the new boosters. The agency is asking the companies to initially submit only the results of tests on mice. Regulators will rely on those results, along with the human neutralizing antibody data from the BA.1 bivalent booster studies, to decide whether to authorize the boosters. The companies will continue to gather more data from human studies; those results probably won't be available until late October or early November. But the big concern is the boosters may not work as well as the mouse data might suggest. Mouse experiments are notoriously unreliable. And with the government telling people not to get the old boosters now and rejecting the first bivalent vaccines, the FDA really needs good evidence that the BA.4/5 boosters are in fact better, critics say. "We need to make sure that we have solid immunogenicity data in people to show that you have a dramatically greater neutralizing antibody response against BA.4, BA.5," says Dr. Paul Offit of the University of Pennsylvania, who also advises the FDA. "I think anything short of that is not acceptable." Some also worry that the approach may further erode the long-faltering efforts to persuade people to get boosted. "I think it would be good to have neutralizing antibody data in a small group of humans," says Dr. Monica Gandhi, an infectious disease researcher at the University of California, San Francisco. "Otherwise, extrapolation may be considered too great." But others agree the time constraints mean the country can't wait for more evidence. The billions of people who have gotten Moderna and Pfizer-BioNTech mRNA vaccines show how safe they are, those experts say. The new booster will be identical to the original vaccines except it will contain genetic coding for two versions of the protein the virus uses to infect cells — the protein from the original vaccine and proteins from the BA.4 and BA.5 omicron subvariants. And some scientists say health officials know enough about how vaccines work to start handling the COVID-19 vaccines like the flu vaccines, which are changed every year to try to match whatever strains are likely to be circulating but aren't routinely tested again every year. "We're going to use all of these data that we've learned through not only from this vaccine but decades of viral immunology to say: 'The way to be nimble is that we're going to do those animal studies," says Deepta Bhattacharya, an immunobiologist at the University of Arizona College of Medicine in Tucson. "We're really not going out too far on a limb here." The companies are expected to submit their data to the FDA by the end of the month and the administration hopes to make millions of doses of the new boosters available starting in September.

|
Scooped by
Juan Lama
|
Sanofi and GlaxoSmithKline’s next generation Covid-19 vaccine could be used as a booster, after new data suggested that it elicited a strong immune response against the Omicron variant. The French healthcare company said the “next-generation” booster jab delivered a “strong immune response” against variants of Covid, including Omicron, after two trials. It was safe and well tolerated in the study of 247 people. The vaccine makers said their jab increased antibodies to tackle the BA.1 Omicron strain by 40-fold in participants who had received two messengerRNA shots, the technology used in popular vaccines from Moderna and BioNTech/Pfizer. They reported that the vaccine outperformed a third dose of the current BioNTech/Pfizer jab, eliciting double the number of neutralising antibodies to BA.1 and BA.2. Thomas Triomphe, executive vice-president of Sanofi Vaccines, said Covid kept evolving and waning immunity was likely to lead to the need for additional booster shots, at least in some populations....

|
Scooped by
Juan Lama
|
Today, the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This action will now make a second booster dose of these vaccines available to other populations at higher risk for severe disease, hospitalization and death. Emerging evidence suggests that a second booster dose of an mRNA COVID-19 vaccine improves protection against severe COVID-19 and is not associated with new safety concerns. The agency amended the emergency use authorizations as follows: - A second booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Moderna COVID-19 Vaccine may be administered to individuals 50 years of age and older at least 4 months after receipt of a first booster dose of any authorized or approved COVID-19 vaccine.
- A second booster dose of the Pfizer-BioNTech COVID-19 Vaccine may be administered to individuals 12 years of age and older with certain kinds of immunocompromise at least 4 months after receipt of a first booster dose of any authorized or approved COVID-19 vaccine. These are people who have undergone solid organ transplantation, or who are living with conditions that are considered to have an equivalent level of immunocompromise.
- A second booster dose of the Moderna COVID-19 Vaccine may be administered at least 4 months after the first booster dose of any authorized or approved COVID-19 vaccine to individuals 18 years of age and older with the same certain kinds of immunocompromise.
“Current evidence suggests some waning of protection over time against serious outcomes from COVID-19 in older and immunocompromised individuals. Based on an analysis of emerging data, a second booster dose of either the Pfizer-BioNTech or Moderna COVID-19 vaccine could help increase protection levels for these higher-risk individuals,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Additionally, the data show that an initial booster dose is critical in helping to protect all adults from the potentially severe outcomes of COVID-19. So, those who have not received their initial booster dose are strongly encouraged to do so.” Today’s action applies only to the Pfizer-BioNTech and Moderna COVID-19 vaccines and the authorization of a single booster dose for other age groups with these vaccines remains unchanged. The agency will continue to evaluate data and information as it becomes available when considering the potential use of a second booster dose in other age groups. The FDA-authorized Pfizer-BioNTech COVID-19 Vaccine and the FDA-approved Comirnaty can be used to provide the authorized booster dose(s). Similarly, the FDA-authorized Moderna COVID-19 Vaccine and the FDA-approved Spikevax are authorized to provide the authorized booster dose(s). Information to Support Authorization of a Second COVID-19 Booster Dose The FDA has determined that the known and potential benefits of a second COVID-19 vaccine booster dose with either of these vaccines outweigh their known and potential risks in these populations. The evidence considered for authorization of a second booster dose following primary vaccination and first booster dose included safety and immune response information provided to the agency as well as additional information on effectiveness submitted by the companies. A summary of safety surveillance data provided to the FDA by the Ministry of Health of Israel on the administration of approximately 700,000 fourth (second booster) doses of the Pfizer-BioNTech COVID-19 Vaccine given at least 4 months after the third dose in adults 18 years of age and older (approximately 600,000 of whom were 60 years of age or older) revealed no new safety concerns. The safety of Moderna COVID-19 Vaccine, when administered as a second booster dose, is informed by experience with the Pfizer-BioNTech COVID-19 Vaccine and safety information reported from an independently conducted study in which the Moderna COVID-19 Vaccine was administered as a second booster dose to 120 participants 18 years of age and older who had received a two-dose primary series and a first booster dose of Pfizer-BioNTech COVID-19 Vaccine at least 4 months prior. No new safety concerns were reported during up to three weeks of follow up after the second booster dose. Immunogenicity data from an ongoing, open-label, non-randomized clinical study in healthcare workers at a single center in Israel were reported in a publication provided to the FDA. In this study, individuals 18 years of age and older who had received primary vaccination and a first booster dose with Pfizer-BioNTech COVID-19 Vaccine were administered a second booster dose of Pfizer-BioNTech COVID-19 Vaccine (154 individuals) or Moderna COVID-19 Vaccine (120 individuals) at least four months after the first booster dose. Among these individuals, increases in neutralizing antibody levels against SARS-CoV-2 virus, including delta and omicron variants were reported two weeks after the second booster as compared to 5 months after the first booster dose. The amendments to the EUAs to include a second booster dose for these populations were granted to Pfizer Inc. and ModernaTX Inc.

|
Scooped by
Juan Lama
|
A new study found that booster protection against symptomatic Omicron fades within 10 weeks. New data from Britain suggests that booster protection against symptomatic Covid caused by the Omicron variant wanes within 10 weeks. There have not yet been enough severe cases of Omicron to calculate how well boosters protect against severe disease, but experts believe the shots will continue to provide significant protection against hospitalization and death. “It will be a few weeks before effectiveness against severe disease with Omicron can be estimated,” the new report, from Britain’s Health Security Agency, noted. “However, based on experience with previous variants, this is likely to be substantially higher than the estimates against symptomatic disease.” In the weeks since Omicron was discovered, multiple studies have suggested that the variant is skilled at evading the antibodies that are produced after vaccination or after infection with the coronavirus. The new report from Britain, which included data on people who had received the AstraZeneca, Pfizer or Moderna shots, confirmed that the vaccines — both the initial two-shot series and booster doses — were less effective and waned faster against Omicron than against Delta. Among people who received two doses of the AstraZeneca vaccine, a booster with one of the mRNA vaccines, made by Pfizer and Moderna, was 60 percent effective at preventing symptomatic disease two to four weeks after the shot. After 10 weeks, however, the Pfizer booster was just 35 percent effective. The Moderna booster was 45 percent effective at up to nine weeks. (The AstraZeneca vaccine is not authorized in the United States, but the Johnson & Johnson shot uses a similar technology.) For people who were given three Pfizer doses, vaccine effectiveness dropped from 70 percent one week after the booster to 45 percent after 10 weeks. Pfizer recipients who received a Moderna booster, on the other hand, seemed to fare better; their vaccine regimen remained up to 75 percent effective at up to nine weeks. The report, which was based on an analysis of about 148,000 Delta cases and 68,000 Omicron cases, also included recent data suggesting that Omicron infections are less likely to lead to hospitalizations than Delta infections. The findings should be interpreted cautiously, the agency noted, because there have still not been many Omicron cases, relatively speaking, and the people who have contracted the variant may not be representative of the broader population. The Biden administration has been encouraging all eligible Americans to receive booster shots as Omicron spreads. In a recent interview on WCBS-AM, a New York radio station, Dr. Anthony S. Fauci, the nation’s leading infectious disease doctor, said that officials were monitoring the effectiveness of mRNA boosters against Omicron. “I do think it’s premature, at least on the part of the United States, to be talking about a fourth dose,” he said. Israel is weighing whether to give a fourth shot to its citizens. Some scientists have warned against a fourth shot, noting that there is not yet evidence that it is necessary and that some immune cells might eventually stop responding to the shots if too many doses are given. Report by UK Health Security Agency (December 23, 2021): https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043807/technical-briefing-33.pdf

|
Scooped by
Juan Lama
|
A 50-microgram dose increased antibodies by roughly 37-fold, and a full dose of 100 micrograms was even more powerful, the company said. A booster shot of the Moderna coronavirus vaccine significantly raises the level of antibodies that can thwart the Omicron variant, the company announced on Monday. The news arrives as Omicron rapidly advances across the world, and most coronavirus vaccines seem unable to stave off infection from the highly contagious variant. Moderna’s results show that the currently authorized booster dose of 50 micrograms — half the dose given for primary immunization — increased the level of antibodies by roughly 37-fold, the company said. A full dose of 100 micrograms was even more powerful, raising antibody levels about 83-fold compared with pre-boost levels, Moderna said. Both doses produced side effects comparable to those seen after the two-dose primary series. But the dose of 100 micrograms showed slightly more frequent adverse reactions relative to the authorized 50-microgram dose. The results are based on laboratory tests that do not capture the full range of the body’s immune response against the virus. Although vaccines may not prevent infection from the variant, they are expected to prevent severe illness in the vast majority of people. The data have also not been published or reviewed by independent experts. Moderna said it was preparing a manuscript with the data that would be posted online. The pharmaceutical companies Pfizer and BioNTech announced earlier this month that a booster shot of their vaccine also increased the level of antibodies against Omicron. Moderna tested a third shot of several versions of its vaccine, each in 20 people. Before boosting, all the individuals had low levels of antibodies that can prevent Omicron infection. At Day 29, after receiving a third shot, the 50-microgram and 100-microgram doses of the current vaccine both sharply increased antibody levels. The company also tested “multivalent” booster shots that incorporate mutations seen in the Beta and Delta variants, many of which are also present in Omicron. Those continuing trials each have 300 to 600 people enrolled in them. The 50-microgram and 100-microgram doses of the multivalent boosters increased antibody levels to similarly high levels, Moderna said. Given how quickly Omicron is marching through the world, Moderna said, the company will focus its near-term efforts on extra shots of the original vaccine. It also plans to test a booster shot that is specific to the Omicron variant early next year and to include Omicron in a multivalent booster. “To respond to this highly transmissible variant, Moderna will continue to rapidly advance an Omicron-specific booster candidate into clinical testing in case it becomes necessary in the future,” said Stéphane Bancel, Moderna’s chief executive officer. Moderna Press Release (Dec. 20, 2021): https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Preliminary-Booster-Data-and-Updates-Strategy-to-Address-Omicron-Variant/default.aspx

|
Scooped by
Juan Lama
|
Pfizer and BioNTech said three doses of their Covid-19 vaccine neutralize the Omicron coronavirus variant, according to the results of an initial laboratory study. The data showed a third dose of the vaccine increases neutralizing antibodies by 25-fold compared with two doses, strengthening the case for and need for booster shots. The preliminary data suggest three doses provide a similar level of antibodies as is observed after two doses against other variants that emerged before Omicron, the companies said. Two vaccine doses showed a 25-fold reduction in neutralizing antibodies against Omicron compared with other variants, which they said suggested two doses “may not be sufficient” protection. “Although two doses of the vaccine may still offer protection against severe disease caused by the Omicron strain, it’s clear from these preliminary data that protection is improved with a third dose of our vaccine,” Pfizer (ticker: PFE) CEO Albert Bourla said in a statement. The companies added that the development of an Omicron-specific vaccine was progressing and is expected to be available by March 2022, if such an adaptation ends up being needed. “The takeaways from Pfizer’s update underscore our belief that the durability of Pfizer’s vaccine sales for Covid-19 remain underappreciated by the Street,” Cantor Fitzgerald analysts said. They rate the stock as Overweight with a target price of $61, implying an 18% upside to Tuesday’s closing price. Another study, published late Tuesday, suggested that Pfizer’s Covid-19 vaccine offers partial protection against the Omicron variant. The small South African laboratory-based study suggested that the variant escapes antibody immunity induced by the Pfizer-BioNTech vaccine but that “considerable immunity” is retained. “It is likely that lesser vaccine-induced protection against infection and disease would be the result,” said Africa Health Research Institute’s executive director, Prof. Willem Hanekom. “Importantly most vaccinologists agree that the current vaccines will still protect against severe disease and death in the face of Omicron infection,” he added. The study was small, including blood samples from just 12 participants, but it is the first scientific data into the efficacy of vaccines against the Omicron variant. The loss of immune protection is “robust but not complete,” head of research Alex Sigal said, adding that a good booster shot would decrease the risk of infection, especially of more severe disease. The study concluded that previous infection followed by vaccination of booster was likely to provide protection. It has been a volatile couple of weeks for Covid-19 vaccine makers, as investors battle to assess the impact of the Omicron variant while waiting for answers to crucial questions. Moderna (MRNA), Pfizer and Novavax (NVAX) were among the sharpest fallers in U.S. premarket trading on Wednesday. That is after plunging Monday and rallying on Tuesday. But Pfizer stock reversed course following the study results and was trading 0.4% higher. Press release by Pfizer (Dec. 8, 2021) available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration cleared a path for millions more Americans to receive Covid-19 vaccine booster shots, as the nation looks to bolster its defenses and prevent another virus surge. The agency said in a statement on Wednesday that Moderna Inc. vaccine recipients 65 and over can receive a third shot, as can adults 18 and up who are at high risk of severe Covid or with frequent institutional or occupational exposure to the virus that causes the disease. Additionally, all J&J recipients 18 and older are eligible for a booster shot at least two months after receiving their first dose. The agency also allowed each of the available Covid vaccines to be used as a booster dose for eligible individuals following completion of a primary vaccination with a different vaccine. The moves will mean the U.S. has a bigger toolkit to try to limit a potential winter virus rebound. The summer’s delta-variant fueled spike in infections helped increase urgency to make boosters available, and health officials across the U.S. are eager to forestall a rebound in cases that could cripple hospitals and disrupt work and school this winter. FDA officials indicated they would also move quickly to expand eligibility for booster shots as more data become available or if breakthrough cases start to rise in younger adults. “We will not hesitate to drop this age range as we see that that benefit clearly outweighs the risk,” said Peter Marks, the head of the agency’s Center for Biologics Evaluation and Research, during a media briefing following the announcement. The clearances came after a panel of expert advisers to the FDA unanimously backed the Moderna and J&J booster regimens in two days of meetings last week. Regulators have now signed off on boosters for all three coronavirus vaccines available in the U.S. Last month, the FDA said people 65 and over and others who are at heightened risk of severe Covid were eligible for a booster dose of the vaccine developed by Pfizer Inc. and BioNTech SE. Moderna shares climbed 0.8% in after-hours trading in New York, while J&J shares gained 0.4% and Pfizer shares rose 0.2%. U.S.-traded shares of Germany-based BioNTech gained 0.9%. Smaller Dose The Moderna booster shot authorized by the FDA is half the dose that is given in the initial two-shot series, and it should be given at least six months after the initial inoculation, regulators said. The FDA said that a single booster dose of the Pfizer vaccine may be given at least 6 months after completing the primary series to people 18 to 64 with frequent institutional or occupational exposure to the coronavirus. In permitting mixing and matching, the FDA is allowing J&J vaccine recipients to receive an additional dose of any cleared vaccine after two months. Likewise, recipients of Moderna and Pfizer who are eligible for a booster would receive their booster, including J&J’s shot, at least six months after their initial immunization regimen. Marks said during the call with reporters that different combinations produce different antibody levels in the short term, but it isn’t clear what that means in terms of actual long-term protection. Seen as a convenient, effective alternative to two-shot messenger RNA vaccines, J&J’s single-shot immunization has seen far less use in the U.S., in part because it isn’t as effective. The drugmaker has also experienced manufacturing problems that limited the shot’s distribution. The decision to allow mixing will create greater flexibility and is beneficial to global public health, Paul Stoffels, J&J’s chief scientific officer, said in a statement. Before the Moderna and J&J booster shots can be administered, the Centers for Disease Control and Prevention’s Advisory Panel on Immunization Practices will make further recommendations about who should receive them. The panel is scheduled to discuss boosters on Thursday. The next big milestone for the U.S. immunization effort looms next week, when the FDA advisory panel is expected to weigh Pfizer’s proposed Covid vaccine for children ages 5 to 11. If authorized, it could begin to roll out to pediatricians’ offices and drugstores as soon as next month. — With assistance by Jeannie Baumann, and Riley Griffin FDA Press Release (Oct. 20, 2021) available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines

|
Scooped by
Juan Lama
|
Background: While Coronavirus disease 2019 (Covid-19) vaccines are highly effective, breakthrough infections are occurring. Booster vaccinations have recently received emergency use authorization (EUA) for certain populations but are restricted to homologous mRNA vaccines. We evaluated homologous and heterologous booster vaccination in persons who had received an EUA Covid-19 vaccine regimen. Methods: In this phase 1/2 open-label clinical trial conducted at ten U.S. sites, adults who received one of three EUA Covid-19 vaccines at least 12 weeks prior to enrollment and had no reported history of SARS-CoV-2 infection received a booster injection with one of three vaccines (Moderna mRNA-1273 100-mcg, Janssen Ad26.COV2.S 5x1010 virus particles, or Pfizer-BioNTech BNT162b2 30-mcg; nine combinations). The primary outcomes were safety, reactogenicity, and humoral immunogenicity on study days 15 and 29. Results: 458 individuals were enrolled: 154 received mRNA-1273, 150 received Ad26.CoV2.S, and 154 received BNT162b2 booster vaccines. Reactogenicity was similar to that reported for the primary series. Injection site pain, malaise, headache, and myalgia occurred in more than half the participants. Booster vaccines increased the neutralizing activity against a D614G pseudovirus (4.2-76-fold) and binding antibody titers (4.6-56-fold) for all combinations; homologous boost increased neutralizing antibody titers 4.2-20-fold whereas heterologous boost increased titers 6.2-76-fold. Day 15 neutralizing and binding antibody titers varied by 28.7-fold and 20.9-fold, respectively, across the nine prime-boost combinations. Conclusion: Homologous and heterologous booster vaccinations were well-tolerated and immunogenic in adults who completed a primary Covid-19 vaccine regimen at least 12 weeks earlier. Available as preprint in medRxiv (Oct. 13, 2021): https://doi.org/10.1101/2021.10.10.21264827
|



 Your new post is loading...
Your new post is loading...







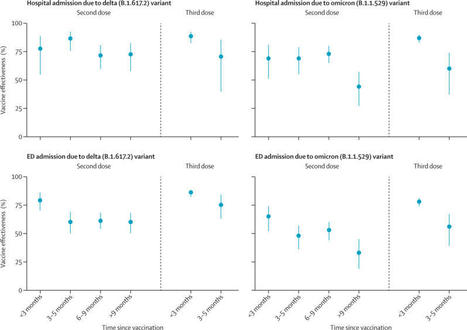
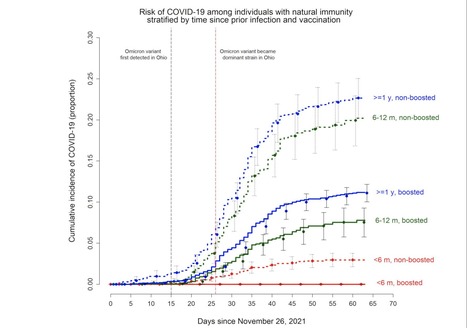

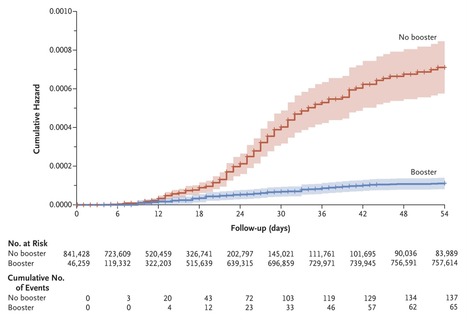


















buy Xanax Alprazolam
Price: $165.0 – $417.0Buy Xanax Online without prescription
Price: $720.0 $247.0Buy yellow xanax bars online
Price: $200.0 – $600.0Buy Zopiclone online uk
Price: $200.0 – $650.0Endocet for sale online
Price: $180.0 – $450.0Order Acxion Fentermina 30mg
Price: $200.0 – $600.0oxycontin for sale online
Price: $200.0 – $1,000.0phentermine for sale online
tramadol for sale online
Price: $100.0 – $900.0Where to buy Ecstasy Online