Your new post is loading...

|
Scooped by
Juan Lama
|
The goal of any vaccine is to induce long-lived plasma cells (LLPC) to provide life-long protection. Natural infection by influenza, measles, or mumps viruses generates bone marrow (BM) LLPC similar to tetanus vaccination which affords safeguards for decades. Although the SARS-CoV-2 mRNA vaccines protect from severe disease, the serologic half-life is short-lived even though SARS-CoV-2-specific plasma cells can be found in the BM. To better understand this paradox, we enrolled 19 healthy adults at 1.5-33 months after SARS-CoV-2 mRNA vaccine and measured influenza-, tetanus-, or SARS-CoV-2-specific antibody secreting cells (ASC) in LLPC (CD19−) and non-LLPC (CD19+) subsets within the BM. All individuals had IgG ASC specific for influenza, tetanus, and SARS-CoV-2 in at least one BM ASC compartment. However, only influenza- and tetanus-specific ASC were readily detected in the LLPC whereas SARS-CoV-2 specificities were mostly excluded. The ratios of non-LLPC:LLPC for influenza, tetanus, and SARS-CoV-2 were 0.61, 0.44, and 29.07, respectively. Even in five patients with known PCR-proven history of infection and vaccination, SARS-CoV-2-specific ASC were mostly excluded from the LLPC. These specificities were further validated by using multiplex bead binding assays of secreted antibodies in the supernatants of cultured ASC. Similarly, the IgG ratios of non-LLPC:LLPC for influenza, tetanus, and SARS-CoV-2 were 0.66, 0.44, and 23.26, respectively. In all, our studies demonstrate that rapid waning of serum antibodies is accounted for by the inability of mRNA vaccines to induce BM LLPC. Preprint available in medRxiv (March 5, 2024): https://doi.org/10.1101/2024.03.02.24303242

|
Scooped by
Juan Lama
|
Once dismissed as less effective, the vaccine now seems to be preventing infections and illness about as well as the two mRNA options. Roughly 17 million Americans received the Johnson & Johnson Covid vaccine, only to be told later that it was the least protective of the options available in the United States. But new data suggest that the vaccine is now preventing infections, hospitalizations and deaths at least as well as the Pfizer-BioNTech and Moderna vaccines. The reasons aren’t clear, and not all experts are convinced that the vaccine has vindicated itself. But the accumulating data nonetheless offer considerable reassurance to recipients of the vaccine and, if confirmed, have broad implications for its deployment in parts of the world. In Africa, for example, distribution of a single-dose vaccine that can be refrigerated for months is by far the most practical option. Johnson & Johnson has at least temporarily shut down the only plant making usable batches of the vaccine. But the South Africa-based Aspen Pharmacare is gearing up to supply large quantities to the rest of the continent. Only about 13 percent of Africans are fully vaccinated, and only about 1 percent have received a booster dose. “In the setting of Africa, where we have the need to quickly get vaccines out, the single dose is very exciting,” said Linda Gail-Bekker, director of the Desmond Tutu H.I.V. Center at the University of Cape Town, who has studied the vaccine’s effectiveness in South Africa. The Johnson & Johnson vaccine was billed as an attractive option for communities with limited access to health care, including some within the United States, because of its ease of delivery and mild side effects. But it has had a bumpy journey. The shot seemed to produce a weaker initial immune response, and more people who got the single-dose vaccine had breakthrough infections, compared with those who got two doses of Pfizer or Moderna, the mRNA vaccines. In April, federal health officials in the United States and in South Africa paused the J.&J. vaccine’s distribution as they examined reports of a rare blood-clotting disorder in women. Though both countries resumed the rollouts soon after, the vaccine’s reputation never fully recovered. But the notion that the vaccine is inferior has grown outdated, some experts said: More recent data suggest that it has more than held its own against its competitors. “We’ve been aware that J.&J. has been kind of downgraded in people’s minds,” Dr. Gail-Bekker said. But “it punches above its weight for a single-dose vaccine.” Until last June, the cumulative data from the C.D.C. showed that immunization with the Moderna vaccine resulted in the lowest rates of breakthrough infections; those who got Johnson & Johnson saw the highest rates, with Pfizer-BioNTech somewhere in the middle. During the summer months, the gaps — particularly between J.&J. and Pfizer — began to narrow. By now, all the vaccines seem to be performing about equally well against coronavirus infections; in fact, Johnson & Johnson appears to be holding up slightly better. As of Jan. 22, the latest data available, unvaccinated people were 3.2 times as likely to become infected as those who received the single-dose Johnson & Johnson vaccine; they were 2.8 times as likely to become infected as those who received two doses of the Moderna vaccine and 2.4 times as likely as those with two doses of Pfizer-BioNTech. Overall, then, the Johnson & Johnson vaccine appeared to be somewhat more protective against infection than the two alternatives. Among Americans who got booster doses, all the vaccines appeared to have roughly the same effectiveness against infection. A booster shot did not add much to Johnson & Johnson’s previous level of protection (although the data do not indicate who received which type of booster shot). The data were collected by the C.D.C. from 29 jurisdictions, representing 67 percent of the population....

|
Scooped by
Juan Lama
|
New studies are boosting assessments that immunity to COVID-19 lasts at least 6-8 months after recovery from the disease. Research published in Science Immunology this week examined 25 patients recovering from the illness. Though antibodies — the immune system proteins that attack virus particles — began dropping in blood samples some 20 days after symptoms appeared, memory B cells that produce antibodies continued to rise in the blood for 150 days and remained high until the 240-day point. This signals subjects’ bodies were primed to fight off the virus for some eight months. Meanwhile, researchers in two other studies found that people who made antibodies to the coronavirus were much less likely to test positive again for up to six months and maybe longer The results bode well for vaccines, which provoke the immune system to make antibodies. A study published Wednesday by the New England Journal of Medicine involved more than 12,500 health workers at Oxford University Hospitals in the United Kingdom. Among the 1,265 who had coronavirus antibodies at the outset, only two had positive results on tests to detect active infection in the following six months and neither developed symptoms. That contrasts with the 11,364 workers who initially did not have antibodies; 223 of them tested positive for infection in the roughly six months that followed. A third study by the National Cancer Institute study involved more than 3 million people who had antibody tests from two private labs in the United States. Only 0.3% of those who initially had antibodies later tested positive for the coronavirus, compared with 3% of those who lacked such antibodies. The results showed that people with antibodies from natural infections were “at much lower risk… on the order of the same kind of protection you’d get from an effective vaccine,” of getting the virus again, said Dr. Ned Sharpless, director of the US National Cancer Institute. “It’s very, very rare” to get reinfected, he said. The institute’s study had nothing to do with cancer — many federal researchers have shifted to coronavirus work because of the pandemic. “It’s very gratifying” to see that the Oxford researchers saw the same risk reduction — 10 times less likely to have a second infection if antibodies were present, Sharpless said. His institute’s report was posted on a website scientists use to share research and is under review at a major medical journal. The findings are “not a surprise … but it’s really reassuring because it tells people that immunity to the virus is common,” said Joshua Wolf, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis who had no role in either study. “We don’t know how long-lasting this immunity is,” Wolf added. Cases of people getting COVID-19 more than once have been confirmed, so “people still need to protect themselves and others by preventing reinfection.” Findings Published in Science Immunology (Dec. 22, 2020): https://doi.org/10.1126/sciimmunol.abf8891 NEJM (DEc. 23, 2020): https://doi.org/10.1056/NEJMoa2034545

|
Scooped by
Juan Lama
|
Antibody levels stayed elevated in the 90 days after people received the second dose of Moderna’s COVID-19 vaccine, raising hopes that the prophylactic can provide protection for one year. With Moderna and Pfizer, in partnership with BioNTech, showing mRNA vaccines provide protection against symptomatic COVID-19 in the short term, attention has turned to durability. The full picture of the durability of protection will only become clear over time, but immunogenicity data from the phase 1/2 trial of Moderna’s mRNA-1273 offer encouragement. In a letter to The New England Journal of Medicine, scientists at the National Institute of Allergy and Infectious Diseases and other research centers presented updated data on 34 people who received the 100-μg dose of mRNA-1273 in the early-phase trial. The phase 3 trial used the same 100-μg dose. The researchers tracked a slight decline in levels of binding and neutralizing antibodies over the 90 days after the administration of the second dose. However, the geometric mean titers for both types of antibody remained above the median of a panel of 41 convalescent COVID-19 patients. The median time from diagnosis of contributors to the convalescent panel was 34 days. Researchers are yet to show what immune characteristics an individual needs to be protected against the coronavirus, limiting our ability to infer the real-world consequences of the antibody increases associated with any vaccine. However, the authors of the NEJM letter said the data show mRNA-1273 “has the potential to provide durable humoral immunity.” Analysts concurred with that assessment. In a note to investors, analysts at Jefferies wrote, “mRNA-1273 induced neutralizing antibody levels stay relatively high and decline only modestly over the initial 3 months suggesting the trajectory of protection from symptomatic disease could last for at least 6 mos (though unclear), so an annual boost would seem good." The NEJM update also touched on the safety of the vaccine. No serious adverse events were seen in the small trial, and no new adverse events deemed related to the vaccine occurred after Day 57. Moderna shared details of the NEJM letter alongside an update on its effort to scale up production. As previously disclosed, Moderna expects to make around 20 million doses available in the U.S. by the end of the year. That early delivery is set to be followed by the shipment of up to 125 million vaccines globally in the first quarter. The U.S. will get around 80% of the vaccines available in the first quarter... Research cited published in NEJM (December 3, 2020): https://doi.org/10.1056/NEJMc2032195
|

|
Scooped by
Juan Lama
|
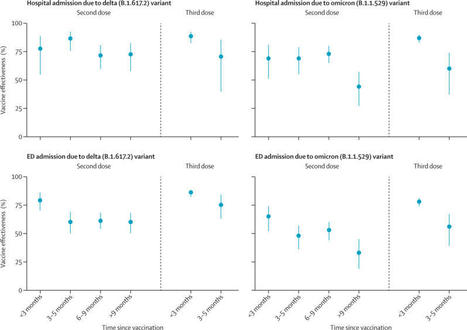
Background The duration of protection against the omicron (B.1.1.529) variant for current COVID-19 vaccines is not well characterised. Vaccine-specific estimates are especially needed. We aimed to evaluate the effectiveness and durability of two and three doses of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine against hospital and emergency department admissions due to the delta (B.1.617.2) and omicron variants. Methods In this case–control study with a test-negative design, we analysed electronic health records of members of Kaiser Permanente Southern California (KPSC), a large integrated health system in California, USA, from Dec 1, 2021, to Feb 6, 2022. Vaccine effectiveness was calculated in KPSC patients aged 18 years and older admitted to hospital or an emergency department (without a subsequent hospital admission) with a diagnosis of acute respiratory infection and tested for SARS-CoV-2 via PCR. Adjusted vaccine effectiveness was estimated with odds ratios from adjusted logistic regression models. This study is registered with ClinicalTrials.gov (NCT04848584). Findings Analyses were done for 11 123 hospital or emergency department admissions. In adjusted analyses, effectiveness of two doses of the BNT162b2 vaccine against the omicron variant was 41% (95% CI 21–55) against hospital admission and 31% (16–43) against emergency department admission at 9 months or longer after the second dose. After three doses, effectiveness of BNT162b2 against hospital admission due to the omicron variant was 85% (95% CI 80–89) at less than 3 months but fell to 55% (28–71) at 3 months or longer, although confidence intervals were wide for the latter estimate. Against emergency department admission, the effectiveness of three doses of BNT162b2 against the omicron variant was 77% (72–81) at less than 3 months but fell to 53% (36–66) at 3 months or longer. Trends in waning against SARS-CoV-2 outcomes due to the delta variant were generally similar, but with higher effectiveness estimates at each timepoint than those seen for the omicron variant. Interpretation Three doses of BNT162b2 conferred high protection against hospital and emergency department admission due to both the delta and omicron variants in the first 3 months after vaccination. However, 3 months after receipt of a third dose, waning was apparent against SARS-CoV-2 outcomes due to the omicron variant, including hospital admission. Additional doses of current, adapted, or novel COVD-19 vaccines might be needed to maintain high levels of protection against subsequent waves of SARS-CoV-2 caused by the omicron variant or future variants with similar escape potential. Published (April 22, 2022) in The Lancet Respiratory Medicine:

|
Scooped by
Juan Lama
|
Researchers at the University of North Carolina and the Fred Hutchinson Cancer Research Center in Washington have developed a way to evaluate vaccines' long-term efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19). The team conducted a series of simulation studies mimicking the trial of the Pfizer/BioNTech vaccine BNT162b2 to investigate the duration of protection under various blinded and unblinded crossover designs. Dan-Yu Lin and colleagues recommend the "rolling crossover" design, where participants that were previously placebo recipients are vaccinated in a timely manner. Under this design, the team's approach can be used to determine when a booster vaccination would be required. This information is also useful for modeling the impact of COVID-19 vaccines at the population level. The approach only requires minimal assumptions and can be applied to both blinded and unblinded crossover designs, irrespective of the length of further follow-up. A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review. Interim trial results only provide data on short-term efficacy The large-scale administration of vaccines that provide long-term protection against SARS-CoV-2 infection would help bring the COVID-19 pandemic to a halt. Interim results from a number of phase 3 clinical trials have demonstrated vaccine efficacy that far exceeds the thresholds required by the World Health Organization and US Food and Drug Administration. Given that vaccine efficacy can wane over time as immune memory declines and viral antigens change, it is crucial to establish how long protection lasts and when booster doses may be required, says the team. How might long-term vaccine efficacy be estimated? Once a vaccine receives Emergency Use Authorization (EUA), placebo-controlled data is still collected in ongoing trials for as long as possible, but at some point, all placebo recipients should be offered the vaccine. Under one approach called "rolling crossover," placebo participants are vaccinated at around the same time that general population members of the same priority tier are vaccinated. The researchers have now shown how to estimate long-term vaccine efficacy in trials that apply this staggered vaccination under both blinded and unblinded crossover strategies. The method fully adjusts for staggered enrollment, change of background incidence over time, and the prioritization of higher-risk individuals during the crossover process. How effective is the approach? The approach provides an unbiased estimation for the entire curve of placebo-controlled vaccine efficacy as a function of time passed since vaccination, up to the point where most placebo recipients have been vaccinated. In a series of simulation studies of the BNT162b2 vaccine trial, the researchers considered 40,000 participants who had been randomly assigned to vaccine or placebo over the course of 4 months. Following approval of the vaccine during the 5th month based on interim results, participants underwent staggered vaccination over the following 5 months. The outcome of interest was time to development of symptomatic COVID-19. The estimated curve of time-varying vaccine efficacy could be used to determine when a booster vaccination would be required to ensure sustained protection. This information is also important for mathematical modeling of the impact COVID-19 vaccines have at the population level..... Cites research available in medRxiv (Jan.21, 2021): https://doi.org/10.1101/2021.01.13.21249779

|
Scooped by
Juan Lama
|
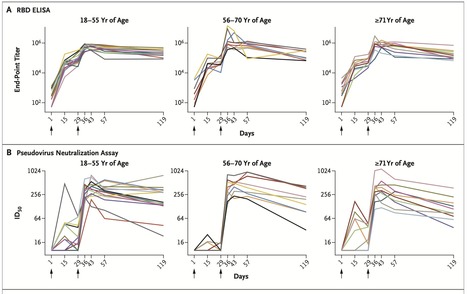
We recently reported the results of a phase 1 trial of a messenger RNA vaccine, mRNA-1273, to prevent infection with SARS-CoV-2; those interim results covered a period of 57 days after the first vaccination. Here, we describe immunogenicity data 119 days after the first vaccination (90 days after the second vaccination) in 34 healthy adult participants in the same trial who received two injections of vaccine at a dose of 100 μg. The injections were received 28 days apart. The recipients were stratified according to age (18 to 55 years, 56 to 70 years, or ≥71 years), and the assays used have been described previously. At the 100-μg dose, mRNA-1273 produced high levels of binding and neutralizing antibodies that declined slightly over time, as expected, but they remained elevated in all participants 3 months after the booster vaccination. Binding antibody responses to the spike receptor–binding domain were assessed by enzyme-linked immunosorbent assay. At the day 119 time point, the geometric mean titer (GMT) was 235,228 (95% confidence interval [CI], 177,236 to 312,195) in participants 18 to 55 years of age, 151,761 (95% CI, 88,571 to 260,033) in those 56 to 70 years of age, and 157,946 (95% CI, 94,345 to 264,420) in those 71 years of age or older Published in NEJM (Dec. 3, 2020): https://doi.org/10.1056/NEJMc2032195
|



 Your new post is loading...
Your new post is loading...