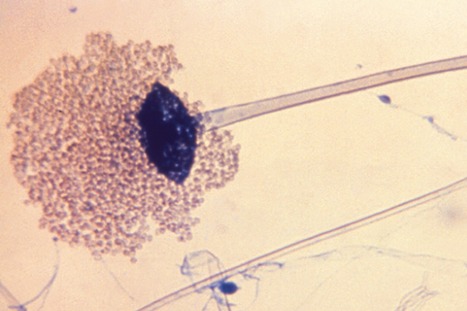
Researchers have cautioned clinicians to be alert for COVID-19–related mucormycosis after the Arkansas Department of Health received reports of 10 cases treated in the state last summer and fall. The cases occurred during a Delta variant surge. Mucormycosis is a rare, life-threatening fungal infection that usually affects people who are immunocompromised or those with uncontrolled diabetes. India, Honduras, and several other countries have reported COVID-19–related mucormycosis cases. COVID-19 can impair immune responses, as can treatment with corticosteroids, increasing mucormycosis risk. Arkansas typically has 9 mucormycosis cases per year, the researchers noted. But during the 2.5-month Delta surge, 6 hospitals in the state reported the 10 cases. Six of the patients died. None of the patients were vaccinated against COVID-19. One was an organ transplant recipient; another had recently had a traumatic injury that developed into an infection. Eight had diabetes, with an average hemoglobin A1c level of 8.6%. Four patients had infection-related nasal and eye symptoms, including 3 who also had brain involvement. Three patients had pulmonary symptoms; 2 had symptoms affecting multiple body systems; and 1 had gastrointestinal symptoms.
“Because of the severity of mucormycosis, it is important that clinicians maintain a high index of suspicion for COVID-19–associated mucormycosis, including in patients without severe immunocompromising conditions,” the researchers wrote in the Morbidity and Mortality Weekly Report. A global systematic review found that mucormycosis symptoms develop about 15 days after a COVID-19 diagnosis and that about one-third of the patients die. Global guidelines recommend urgent surgical and systemic antifungal treatment for patients with mucormycosis. To help prevent infections, the authors recommend that patients with diabetes maintain glycemic control and be vaccinated against COVID-19. They also recommend following the National Institutes of Health’s guidelines for judicious corticosteroid use when treating COVID-19.
Published in JAMA Network (Feb. 8, 2022):



 Your new post is loading...
Your new post is loading...