Your new post is loading...

|
Scooped by
Juan Lama
|
The COVID-19 pandemic has limited the access of patients with cardiovascular diseases to healthcare services, causing excess deaths. However, a detailed analysis of temporal variations of excess cardiovascular mortality during the COVID-19 pandemic has been lacking. Here we estimate time-varied excess cardiovascular deaths (observed deaths versus expected deaths predicted by the negative binomial log-linear regression model) in the United States. From March 2020 to March 2022 there were 90,160 excess cardiovascular deaths, or 4.9% more cardiovascular deaths than expected. Two large peaks of national excess cardiovascular mortality were observed during the periods of March–June 2020 and June–November 2021, coinciding with two peaks of COVID-19 deaths, but the temporal patterns varied by state, age, sex and race and ethnicity. The excess cardiovascular death percentages were 5.7% and 4.0% in men and women, respectively, and 3.6%, 8.8%, 7.5% and 7.7% in non-Hispanic White, Black, Asian and Hispanic people, respectively. Our data highlight an urgent need for healthcare services optimization for patients with cardiovascular diseases in the COVID-19 era. Ran et al. report that excess cardiovascular mortality in the United States during the first two years of the COVID-19 pandemic coincided with the waves of COVID-19 deaths and was demographically diverse. Published in Nature Cardiovascular Reserch (Feb. 27, 2023): https://doi.org/10.1038/s44161-023-00220-2

|
Scooped by
Juan Lama
|
New data analysis from the Smidt Heart Institute at Cedars-Sinai found that deaths from heart attacks rose significantly during pandemic surges, including the COVID-19 Omicron surges, overall reversing a heart-healthier pre-pandemic trend. Prior to the COVID-19 pandemic, heart attacks were the leading cause of death worldwide but were steadily on the decline. However, the new study—recently published in the Journal of Medical Virology—shows that heart attack death rates took a sharp turn and increased for all age groups during the pandemic. The spikes in heart attack deaths have tracked with surges of COVID-19 infection—even during the presumed less-severe omicron phase of the pandemic. Furthermore, the data showed the increase was most significant among individuals ages 25-44, who are not usually considered at high risk for heart attack. "The dramatic rise in heart attacks during the pandemic has reversed what was a prior decadelong steady improvement in cardiac deaths," said Yee Hui Yeo, MD, first author of the study and a Cedars-Sinai physician-scientist. "We are still learning the many ways by which COVID-19 affects the body, regardless of age, gender, ethnicity or race." Using data from the Centers for Disease Control and Prevention's National Vital Statistics System, the Cedars-Sinai researchers identified 1,522,699 deaths from heart attacks—medically called acute myocardial infarctions—between April 1, 2012, and March 31, 2022. Investigators then compared age-related mortality rates between pre-pandemic and pandemic periods, as well as demographic groups and regions. Key findings from the study include: - In the year before the pandemic, there were 143,787 heart attack deaths; within the first year of the pandemic, this number had increased by 14% to 164,096.
- The excess in acute myocardial infarction-associated mortality has persisted throughout the pandemic, even during the most recent period marked by a surge of the presumed less-virulent omicron variant.
- Researchers found that although acute myocardial infarction deaths during the pandemic increased across all age groups, the relative rise was most significant for the youngest group, ages 25 to 44.
- By the second year of the pandemic, the "observed" compared to "predicted" rates of heart attack death had increased by 29.9% for adults ages 25-44, by 19.6% for adults ages 45-64, and by 13.7% for adults age 65 and older.
"There are several potential explanations for the rapid rise in cardiac deaths in patients with COVID-19, yet still many unanswered questions," said Yeo. "Importantly, our results highlight disparities in mortality that have emerged from the COVID-19 pandemic and that are persisting even through the omicron era." The possible explanations, Yeo said, include that COVID-19 may trigger or accelerate the presentation of preexisting coronary artery disease, even in younger adults. Reasons for the spike in heart-related conditions could also be related to psychological and social challenges associated with the pandemic, including job loss and other financial pressures that can cause acute or chronic stress leading to cardiac disease. The research team members say they have long known that infections such as the flu can increase risk for heart disease and heart attack, but the sharp rise in heart attack deaths is like nothing seen before. "There is something very different about how this virus affects the cardiac risks," said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the Department of Cardiology at the Smidt Heart Institute and senior and co-corresponding author of the study. "The difference is likely due to a combination of stress and inflammation, arising from predisposing factors and the way this virus biologically interacts with the cardiovascular system." Yeo, Cheng and the broader Smidt Heart Institute team hope that greater awareness and more research will expand the medical community's ability to manage and mitigate these risks. Research cited published in J. Med. Virology (Sept. 29, 2022): https://doi.org/10.1002/jmv.28187

|
Scooped by
Juan Lama
|
BACKGROUND Reports have suggested an association between the development of myocarditis and the receipt of messenger RNA (mRNA) vaccines against coronavirus disease 2019 (Covid-19), but the frequency and severity of myocarditis after vaccination have not been extensively explored. METHODS We searched the database of Clalit Health Services, the largest health care organization (HCO) in Israel, for diagnoses of myocarditis in patients who had received at least one dose of the BNT162b2 mRNA vaccine (Pfizer–BioNTech). The diagnosis of myocarditis was adjudicated by cardiologists using the case definition used by the Centers for Disease Control and Prevention. We abstracted the presentation, clinical course, and outcome from the patient’s electronic health record. We performed a Kaplan–Meier analysis of the incidence of myocarditis up to 42 days after the first vaccine dose. RESULTS Among more than 2.5 million vaccinated HCO members who were 16 years of age or older, 54 cases met the criteria for myocarditis. The estimated incidence per 100,000 persons who had received at least one dose of vaccine was 2.13 cases (95% confidence interval [CI], 1.56 to 2.70). The highest incidence of myocarditis (10.69 cases per 100,000 persons; 95% CI, 6.93 to 14.46) was reported in male patients between the ages of 16 and 29 years. A total of 76% of cases of myocarditis were described as mild and 22% as intermediate; 1 case was associated with cardiogenic shock. After a median follow-up of 83 days after the onset of myocarditis, 1 patient had been readmitted to the hospital, and 1 had died of an unknown cause after discharge. Of 14 patients who had left ventricular dysfunction on echocardiography during admission, 10 still had such dysfunction at the time of hospital discharge. Of these patients, 5 underwent subsequent testing that revealed normal heart function. CONCLUSIONS Among patients in a large Israeli health care system who had received at least one dose of the BNT162b2 mRNA vaccine, the estimated incidence of myocarditis was 2.13 cases per 100,000 persons; the highest incidence was among male patients between the ages of 16 and 29 years. Most cases of myocarditis were mild or moderate in severity. (Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.) Published in NEJM (Oct. 6, 2021): https://doi.org/10.1056/NEJMoa2110737

|
Scooped by
Juan Lama
|
The U.S. Centers for Disease Control and Prevention (CDC) has not found a link between heart inflammation and COVID-19 vaccines, the agency's Director Rochelle Walensky said on Tuesday. "We have not seen a signal and we've actually looked intentionally for the signal in the over 200 million doses we've given," Walensky said in a press briefing. She said the CDC is in touch with the U.S. Department of Defense over its investigation of 14 cases of heart inflammation or myocarditis among people who were vaccinated through the military's health services. "It is a different demographic than we normally see and we will be working with DOD to understand what is happening in those 14 cases," Walensky said. The U.S. vaccination program targeted older Americans first, and the concerns over myocarditis are in younger vaccinated people. At least 17.9 million people in the United States under the age of 30 have received one COVID-19 shot, according to CDC data. Israel's Health Ministry said on Sunday it was examining a small number of cases of heart inflammation in people who had received Pfizer's (PFE.N) COVID-19 vaccine, though it had not yet drawn any conclusions. Most of the cases in Israel were reported among people up to age 30. read more Pfizer has said it has not observed a higher rate of the condition than would normally be expected in the general population. COVID-19 itself has been linked to cases of myocarditis in some patients.

|
Scooped by
Juan Lama
|
COVID-19 is now the leading cause of death in the United States topping heart disease, according to The Institute for Health Metrics and Evaluation at the University of Washington’s School of Medicine. IHME says COVID-19 was the cause of nearly 12,000 deaths last week, putting it ahead of ischemic heart disease and lung cancer. As hospital beds fill up governors across the country are weighing new restrictions. Health officials warn the pandemic will likely get worse. However, there is some hope on the horizon. In northern California, people in five Bay Area counties are bracing for new COVID-19 stay at home orders taking effect Sunday night. Health officials hope shutting down outdoor dining playgrounds and salons can quickly save lives. “Until we get through this wave, you should not meet in person with anyone you do not live with, even in a small group, and even outdoors with precautions,” said Dr. Lisa Hernandez, Berkeley California public health officer. But the restrictions have business owners losing hope. “Christmas time they come to get the nail beautiful for the holiday and we shut down again. We don’t know what we’re going to do for our financial,” said nail salon owner, Linda Nguyen. With intensive care unit bed capacity dwindling, the stay at home orders could spread to central and southern California soon as well. Americans are dying from COVID at a rate of nearly two people per minute. The Food and Drug Administration could vote Thursday for Emergency Use Authorization for the first COVID-19 vaccine in the US from drugmaker Pfizer. “As early as Friday of next week we could see vaccinations happening across the country,” said Dr. James Hildreth of the FDA advisory committee. But experts say even if vaccinations start this week, it will be some time before everyone in the US can get one, and before life truly returns to normal. But health officials warn things are bad now, and will get much worse without stronger efforts to stop the virus. Michigan Gov. Gretchen Whitmer says she is waiting on more data from Thanksgiving to determine if the state’s three-week pause will be extended. The number of confirmed cases of the coronavirus (COVID-19) in Michigan has risen to 395,036 as of Saturday, including 9,854 deaths, state officials report. Saturday’s update represents 6,004 new cases and 193 additional deaths, including 145 deaths identified during a review of records -- meaning they did not occur between Friday and Saturday. The state reported a total of 197,750 recoveries on Saturday.

|
Scooped by
Juan Lama
|
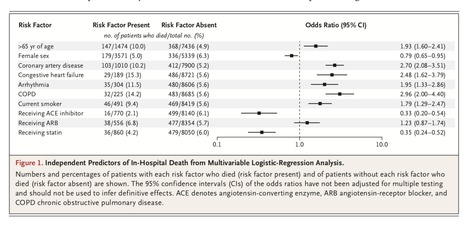
Coronavirus disease 2019 (Covid-19) may disproportionately affect people with cardiovascular disease. Concern has been aroused regarding a potential harmful effect of angiotensin-converting–enzyme (ACE) inhibitors and angiotensin-recep- tor blockers (ARBs) in this clinical context. Using an observational database from 169 hospitals in Asia, Europe, and North America, we evaluated the relationship of cardiovascular disease and drug therapy with in-hospital death among hospitalized patients with Covid-19 who were admit- ted between December 20, 2019, and March 15, 2020, and were recorded in the Surgical Outcomes Collaborative registry as having either died in the hospital or survived to discharge as of March 28, 2020. Of the 8910 patients with Covid-19 for whom discharge status was available at the time of the analysis, a total of 515 died in the hospital (5.8%) and 8395 survived to discharge. The factors we found to be independently associated with an in- creased risk of in-hospital death were an age greater than 65 years (mortality of 10.0%, vs. 4.9% among those ≤65 years of age; odds ratio, 1.93; 95% confidence interval [CI], 1.60 to 2.41), coronary artery disease (10.2%, vs. 5.2% among those without disease; odds ratio, 2.70; 95% CI, 2.08 to 3.51), heart failure (15.3%, vs. 5.6% among those without heart failure; odds ratio, 2.48; 95% CI, 1.62 to 3.79), cardiac arrhythmia (11.5%, vs. 5.6% among those without arrhythmia; odds ratio, 1.95; 95% CI, 1.33 to 2.86), chronic obstructive pulmonary disease (14.2%, vs. 5.6% among those without disease; odds ratio, 2.96; 95% CI, 2.00 to 4.40), and current smoking (9.4%, vs. 5.6% among former smokers or nonsmokers; odds ratio, 1.79; 95% CI, 1.29 to 2.47). No increased risk of in-hospital death was found to be associated with the use of ACE inhibitors (2.1% vs. 6.1%; odds ratio, 0.33; 95% CI, 0.20 to 0.54) or the use of ARBs (6.8% vs. 5.7%; odds ratio, 1.23; 95% CI, 0.87 to 1.74). Our study confirmed previous observations suggesting that underlying cardiovascu- lar disease is associated with an increased risk of in-hospital death among patients hospitalized with Covid-19. Our results did not confirm previous concerns regarding a potential harmful association of ACE inhibitors or ARBs with in-hospital death in this clinical context.... In our analyses, use of either ACE inhibitors or statins was associated with better survival among patients with Covid-19. However, these as- sociations should be considered with extreme cau- tion. Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. Published in New England. J. Medicine (May 1, 2020): https://doi.org/10.1056/NEJMoa2007621
|

|
Scooped by
Juan Lama
|
Scientists are starting to detect clots in long COVID patients’ smallest blood vessels—which might help explain the condition’s debilitating symptoms. For more than two years, scientists have been trying to understand why millions of people across the world are experiencing lingering symptoms despite recovering from their COVID-19 infection. They’ve proposed several hypotheses including the presence of microclots—tiny blood clots that can block capillaries and potentially affect blood and oxygen flow. In a 2021 study, physiologist Etheresia Pretorius at theStellenbosch University in South Africa and her colleagues were the first to suggest that microclots may be linked to this debilitating condition called long COVID. In a follow-up study, she and her colleagues showed that the SARS-CoV-2 spike protein triggers the formation of such clots, which the body’s natural clot-busting process doesn’t seem to break down easily. This finding has led some scientists in the United States, with guidance from Pretorius, to test people with long COVID for microclots. Lisa McCorkell, co-founder of the long COVID-focused Patient-Led Research Collaborative, was thrilled when she heard the news last year. Fluorescence microscopy images reveal microclots circulating in healthy blood (left) and a severe one in a long COVID patient (right). The COVID clots are more resistant to a natural clot-busting process called fibrinolysis and don’t break down easily. Micrograph by Chantelle Venter and Resia Pretorius McCorkell had experienced severe shortness of breath, extreme fatigue, and brain fog for several months following her mild COVID-19 symptoms in March 2020, when the pandemic began. In August that year, when she started to feel better, McCorkell took a workout class. But a day later, her heart rate spiked, she struggled to breathe, and she rushed to the emergency room. “That lowered my baseline quite a bit,” she says. “Before COVID, I was running half marathons, so it was a very dramatic change.” In December 2020, the 28-year-old finally came to terms with how sick she was and that her illness wasn’t temporary. In late 2021, her suspicions were confirmed when she was diagnosed with postural orthostatic tachycardia syndrome (POTS), a condition documented in several long COVID patientsthat can disrupt breathing and cause heart palpitations and dizziness on standing up. POTS has no cure and some patients, including McCorkell, manage symptoms by increasing fluids and salt intake. But a year after her diagnosis she still suffers post-exertional malaise that worsens these symptoms. What’s frustrating for McCorkell and many other long COVID patients is blood and other routine tests turn up normal despite their debilitating condition. In November 2022, she flew from California to New York where David Putrino, a rehabilitation and long COVID scientist at Mount Sinai Health System, and his collaborators are collecting blood samples to search for microclots. “We’re very early,” Putrino says. “We’ve only tested a few dozen folks so far.” But every sample from long COVID patients, including McCorkell’s, has revealed such clots. When she first saw the microscope images of fluorescent green blobs revealing the microclots, she cried with relief. For her, the confirmation that she has microclots felt like validation of her illness, “especially after not getting a PCR test at the beginning and being gaslit throughout the last few years.” While some experts agree the microclots hypothesis is plausible, they think it could be just one piece of the long COVID puzzle. But they want to see more research that demonstrates how these clots contribute to long COVID symptoms and whether getting rid of them leads to improved health outcomes. How microclots form Unlike blood clots that block arteries or veins, microclots occur in small blood vessels. They form when a soluble protein called fibrinogen is exposed to inflammation-causing molecules, which can bind to the fibrinogen and aggregate into sticky blobs. “They are not capable of clogging large vessels; they’re not capable of causing life-threatening symptoms,” Putrino says, but notes, “They can significantly affect organ function.” Pretorius and her colleagues have been studying such microclots for more than a decade and have observed them in patients with type 2 diabetes, chronic fatigue syndrome, Alzheimer’s, and Parkinson’s disease. In a preliminary 2021 study, they saw substantial microclot formation in the blood of acute COVID-19 patients, as well as people with long COVID who experience persistent symptoms for six months or longer. “The main difference between microclots we find in diabetes and other conditions is that they break up quite easily,” Pretorius says. COVID microclots are harder to disintegrate. Trapped inside the persistent microclots, her team found high levels of inflammatory molecules and a protein called alpha 2-antiplasmin that prevents their breakdown. Such blockages in tiny blood vessels throughout the body could hinder the supply of oxygen and nutrients to the organs and tissues, potentially leading to long COVID symptoms like fatigue, muscle pain, and brain fog. But what’s triggering the microclots formation? Pretorius and her colleagues think it’s the SARS-CoV-2 spike protein, which can linger in the blood of long COVID patients for up to a year. In a 2021 study, the team added spike proteins to healthy blood and were able to trigger the development of microclots. They also found that in the presence of the spike, the microclots were more resistant to fibrinolysis—a natural process that enables the removal of clots. “Our belief is that the spike protein binds to the healthy fibrinogen,” Pretorius says. “We think that interaction perhaps makes for a tighter [microclot] structure and a bigger structure.” If these microclots persist for prolonged periods, the body could produce autoantibodies—proteins that inadvertently attack the body’s own healthy tissues and cause debilitating disorders. “It’s those individuals who we are particularly worried about,” she says. How scientists detect microclots Detecting microclots requires a specialized laboratory technique called fluorescence microscopy. “You can’t just go to the doctor’s office and get tested for microclots,” says microbiologist Amy Proal, of the nonprofit PolyBio Research Foundation and co-founder of the long COVID Research Initiative. The process involves drawing blood, spinning it, and adding a fluorescent agent to see the clots under a fluorescence microscope. It’s not a widely available tool in general pathology labs. But what’s unknown is the sensitivity and specificity of this method. “If you’ve got 500 long COVID patients, is this assay positive 100 percent of the times or 20 percent,” asks hematologist Jeffery Laurence at the Weill Cornell Medical College in New York City, who isn’t involved in Putrino’s or Pretorius’s research. “Given that a similar phenomenon occurs in other diseases, how specific is this for COVID.” He also points out that published microclot studies have been done in a small number of long COVID patients, but future work should involve testing blood samples from many more people and replicating the research in several labs. Putrino, in collaboration with immunologist Akiko Iwasaki, at Yale University, plans to test hundreds of long COVID patients “because a few dozens is by no means valid for saying everybody [with long COVID] has microclots,” he says. For now, Putrino and his team are seeing a correlation between the number of microclots on a microscope slide and the severity of a patient’s cognitive impairment. These include their ability to regulate emotions, plan and put together long-term solutions to problems, or figure out ways to deal with real-time situations as they’re changing. The research team is also developing an objective measure for microclots. “We’re still at a very rudimentary stage,” Putrino says. Hematologist Yazan Abou-Ismail at the University of Utah, who isn’t associated with the microclots research but finds the theory plausible in the context of long COVID, also hopes to see studies that document what’s happening inside the capillaries and organs of long COVID patients with microclots. “It can be hypothesized that the microclots end up obstructing small blood vessels,” he says, “but we don’t really know whether there’s an actual obstruction.” Treating microclots While researchers try to determine the prevalence of microclots in people with long COVID and why they form, patients are suffering and desperate for treatments. In a December 2021 preprint study, which is yet to peer-reviewed, Pretorius and her team showed a decrease in microclots and reduced platelet activation—a condition that accompanies microclot presence—in 24 long COVID patients who were administered a combination of anticoagulant Apixiban and a dual antiplatelet therapy for a month. However, they’re in the process of revising the study to include more patients and measurements of their health outcomes following the treatment. “But we need clinical trials to show anticoagulation approaches and antiplatelet approaches have efficacy,” Putrino says. He also wonders if clots in small blood vessels may need different anticoagulants compared to those used against large clots. McCorkell, on the other hand, is taking her treatment into her own hands and experimenting with over-the-counter enzyme supplements like serrapeptas and nattokinase that seem to breakdown blood clots but aren’t approved by the U.S. Food and Drug Administration. Like many other people with long COVID, McCorkell is disappointed and angered that there aren’t clinical trials to test the use of such supplements and other off-label therapies that some patients are resorting to for relief. Many health providers are also often unable to help. Although she hasn’t experienced any side effects so far, McCorkell knows of some individuals who have had nausea and vomiting episodes from taking the same supplements. Pretorius and her team plan to conduct a study to test if these supplements are effective, but until then many patients are on their own. “Given the scale of the issue and how much it impacts people’s lives, we need an Operation Warp Speed situation,” McCorkell says. “It’s frustrating that we’re not further along.”

|
Scooped by
Juan Lama
|
Massive study shows a long-term, substantial rise in risk of cardiovascular disease, including heart attack and stroke, after a SARS-CoV-2 infection. Even a mild case of COVID-19 can increase a person’s risk of cardiovascular problems for at least a year after diagnosis, a new study1 shows. Researchers found that rates of many conditions, such as heart failure and stroke, were substantially higher in people who had recovered from COVID-19 than in similar people who hadn’t had the disease. What’s more, the risk was elevated even for those who were under 65 years of age and lacked risk factors, such as obesity or diabetes. “It doesn’t matter if you are young or old, it doesn’t matter if you smoked, or you didn’t,” says study co-author Ziyad Al-Aly at Washington University in St. Louis, Missouri, and the chief of research and development for the Veterans Affairs (VA) St. Louis Health Care System. “The risk was there.” Al-Aly and his colleagues based their research on an extensive health-record database curated by the United States Department of Veterans Affairs. The researchers compared more than 150,000 veterans who survived for at least 30 days after contracting COVID-19 with two groups of uninfected people: a group of more than five million people who used the VA medical system during the pandemic, and a similarly sized group that used the system in 2017, before SARS-CoV-2 was circulating. Troubled hearts People who had recovered from COVID-19 showed stark increases in 20 cardiovascular problems over the year after infection. For example, they were 52% more likely to have had a stroke than the contemporary control group, meaning that, out of every 1,000 people studied, there were around 4 more people in the COVID-19 group than in the control group who experienced stroke. The risk of heart failure increased by 72%, or around 12 more people in the COVID-19 group per 1,000 studied. Hospitalization increased the likelihood of future cardiovascular complications, but even people who avoided hospitalization were at higher risk for many conditions. “I am actually surprised by these findings that cardiovascular complications of COVID can last so long,” Hossein Ardehali, a cardiologist at Northwestern University in Chicago, Illinois, wrote in an e-mail to Nature. Because severe disease increased the risk of complications much more than mild disease, Ardehali wrote, “it is important that those who are not vaccinated get their vaccine immediately”. Ardehali cautions that the study’s observational nature comes with some limitations. For example, people in the contemporary control group weren’t tested for COVID-19, so it’s possible that some of them actually had mild infections. And because the authors considered only VA patients — a group that’s predominantly white and male — their results might not translate to all populations. Ardehali and Al-Aly agree that health-care providers around the world should be prepared to address an increase in cardiovascular conditions. But with high COVID-19 case counts still straining medical resources, Al-Aly worries that health authorities will delay preparing for the pandemic’s aftermath for too long. “We collectively dropped the ball on COVID,” he said. “And I feel we’re about to drop the ball on long COVID.” Published in Nature (Feb. 10, 2022): https://doi.org/10.1038/d41586-022-00403-0

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration said on Wednesday it plans to move quickly to add a warning about rare cases of heart inflammation in adolescents and young adults to fact sheets for the Pfizer/BioNTech (PFE.N), and Moderna (MRNA.O) COVID-19 vaccines. U.S. Centers for Disease Control and Prevention (CDC) advisory groups, meeting to discuss reported cases of the heart condition after vaccination, found the inflammation in adolescents and young adults is likely linked to the vaccines, but that the benefits of the shots appeared to clearly outweigh the risk. Moderna shares closed down 4.2%, while Pfizer fell 1.4%. Health regulators in several countries have been investigating whether the Pfizer/BioNTech and Moderna shots using new mRNA technology present a risk and, if so, how serious. The CDC said that patients with heart inflammation following vaccination generally recover from the symptoms and do well. The U.S. Department of Health And Human Services, joined by leading U.S. doctors groups and public health officials, put out a statement underscoring that the vaccines are safe and effective and that the heart side effect is "extremely rare." "We strongly encourage everyone age 12 and older who are eligible to receive the vaccine under Emergency Use Authorization to get vaccinated," it said. Doctors and hospitals have been warned by the CDC to watch for symptoms of myocarditis or pericarditis, and the FDA warning will further raise awareness. Concerns about the more highly transmissible Delta coronavirus variant taking hold in the United States, and its impact on younger people, have added to the urgency to increase vaccinations even as the inoculation effort here has slowed considerably. read more The number of Americans receiving their first dose of COVID-19 vaccine is down about 85% since peaking in mid-April, and will likely fail to meet President Joe Biden's goal to have delivered at least one shot to 70% of adults by July 4. "Based on the available data, a warning statement in the factsheets for both healthcare providers and vaccine recipients and caregivers would be warranted," FDA official Doran Fink said at the advisory committee meeting. Fink, deputy director of the agency's vaccines division, said the FDA expects to move quickly to add the warning after the meeting concludes. The cases of heart inflammation appear to be notably higher in the week after the second vaccine dose and in males. The CDC identified 309 hospitalizations from the heart inflammation in persons under the age of 30, of which 295 have been discharged. Dr. Tom Shimabukuro, deputy director of the CDC's Immunization Safety Office, said in a presentation that data from one of the agency's safety monitoring systems - Vaccine Safety Datalink (VSD) – suggests a rate of 12.6 cases per million in the three weeks after the second shot in 12- to 39-year-olds. "We're observing this in the younger age groups, mainly in people in the teens and early 20s, and observing it more in males, compared to females," Shimabukuro said. "This effect largely kind of disappears once you get into these older age groups - individuals 50 and over." The CDC has been investigating heart inflammation cases mainly in young men for several months. The Israeli health ministry earlier this month said it saw a possible link between such cases and Pfizer's COVID-19 vaccine. read more The CDC said it was still assessing the risk from the condition and did not specifically confirm a causal relationship between the vaccines and the heart issue. It did, however, say that a much-higher-than expected number of young men between the ages of 12 and 24 have experienced heart inflammation after their second vaccine dose. According to data from the U.S. Vaccine Adverse Event Reporting System (VAERS), there were 347 observed cases of heart inflammation in the week after the second vaccine dose in males aged 12 to 24. That compares with expectations of 12 or fewer cases for males in that age range based on U.S. population background incidence rates, the CDC said. Pfizer, whose vaccine has been authorized for use in Americans as young as 12, previously said it had not observed a higher rate of heart inflammation than would normally be expected in the general population. read more Moderna said it is aware of reports of heart inflammation cases following administration of mRNA vaccines. It said it is working with public health and regulatory authorities to assess the issue. Over 138 million Americans have so far been fully vaccinated with one of the two mRNA vaccines, according to CDC data as of Monday.

|
Scooped by
Juan Lama
|
Using cardiac MRI, researchers found myocardial injury in more than half of patients hospitalized with severe COVID-19 and elevated troponin a few months after hospital discharge. “We found evidence of high rates of heart muscle injury that could be seen on the scans a month or two after discharge. Whilst some of this may have been preexisting, MRI scanning shows that some were new, and likely caused by COVID-19,” Marianna Fontana, MD, PhD, professor of cardiology at University College London, said in a press release. “Importantly, the pattern of damage to the heart was variable, suggesting that the heart is at risk of different types of injury. While we detected only a small amount of ongoing injury, we saw injury to the heart that was present even when the heart’s pumping function was not impaired and might not have been picked up by other techniques. In the most severe cases, there are concerns that this injury may increase the risks of heart failure in the future, but more work is needed to investigate this further.” For this analysis, researchers included 148 patients from six hospitals (mean age, 64 years; 70% men) with severe COVID-19 infection and elevated troponin levels, all requiring hospitalization (32% requiring mechanical ventilation), who underwent convalescent cardiac MRI and adenosine stress perfusion at a median of 68 days after discharge. “Raised troponin levels are associated with worse outcomes in COVID-19 patients. Patients with severe COVID-19 disease often have preexisting heart-related health problems, including diabetes, raised blood pressure and obesity,” Fontana said in the release. “During severe COVID-19 infection, however, the heart may also be directly affected. Unpacking how the heart can become damaged is difficult, but MRI scans of the heart can identify different patterns of injury, which may enable us to make more accurate diagnoses and to target treatments more effectively.” Myocardial injury in COVID-19 Patients with abnormal troponin levels were offered a cardiac MRI after discharge and were compared with a control group of patients who did not have COVID-19 and 40 healthy volunteers. Researchers observed that left ventricular function was normal in 89% of the overall cohort (mean ejection fraction, 67%). Late gadolinium enhancement and/or ischemia was found in 54% of participants hospitalized for severe COVID-19 infection, which included a myocarditis-like scar in 26%, MI and/or ischemia in 22% and dual pathology in 6%. According to the study, myocarditis-like injury was limited to three or fewer myocardial segments in 88% of patients with no LV dysfunction, of which 30% had active myocarditis. In addition, MI was observed in 19% and inducible ischemia in 26% of patients who underwent stress perfusion after discharge. Moreover, of patients with ischemic injury, 66% had no prior history of coronary disease. Researchers found no evidence of diffuse fibrosis or edema in the remote myocardium. Two new opportunities “These findings give us two opportunities,” Fontana said in the release. “Firstly, to find ways of preventing the injury in the first place, and from some of the patterns we have seen, blood clotting may be playing a role, for which we have potential treatments. Secondly, detecting the consequences of injury during convalescence may identify subjects who would benefit from specific supporting drug treatments to protect heart function over time.” Findings published in European Heart Journal (Feb. 18, 2021): https://doi.org/10.1093/eurheartj/ehab075

|
Scooped by
Juan Lama
|
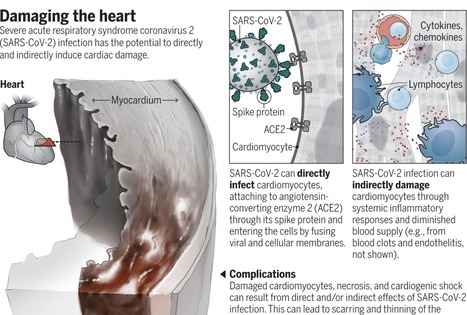
The family of seven known human coronaviruses are known for their impact on the respiratory tract, not the heart. However, the most recent coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has marked tropism for the heart and can lead to myocarditis (inflammation of the heart), necrosis of its cells, mimicking of a heart attack, arrhythmias, and acute or protracted heart failure (muscle dysfunction). These complications, which at times are the only features of coronavirus disease 2019 (COVID-19) clinical presentation, have occurred even in cases with mild symptoms and in people who did not experience any symptoms. Recent findings of heart involvement in young athletes, including sudden death, have raised concerns about the current limits of our knowledge and potentially high risk and occult prevalence of COVID-19 heart manifestations. The four “common cold” human coronaviruses—HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1—have not been associated with heart abnormalities. There were isolated reports of patients with Middle East respiratory syndrome (MERS; caused by MERS-CoV) with myocarditis and a limited number of case series of cardiac disease in patients with SARS (caused by SARS-CoV) (1). Therefore, a distinct feature of SARS-CoV-2 is its more extensive cardiac involvement, which may also be a consequence of the pandemic and the exposure of tens of millions of people to the virus. What appears to structurally differentiate SARS-CoV-2 from SARS is a furin polybasic site that, when cleaved, broadens the types of cells (tropism) that the virus can infect (2). The virus targets the angiotensin-converting enzyme 2 (ACE2) receptor throughout the body, facilitating cell entry by way of its spike protein, along with the cooperation of the cellular serine protease transmembrane protease serine 2 (TMPRSS2), heparan sulfate, and other proteases (3). The heart is one of the many organs with high expression of ACE2. Moreover, the affinity of SARS-CoV-2 to ACE2 is significantly greater than that of SARS (4). The tropism to other organs beyond the lungs has been studied from autopsy specimens: SARS-CoV-2 genomic RNA was highest in the lungs, but the heart, kidney, and liver also showed substantial amounts, and copies of the virus were detected in the heart from 16 of 22 patients who died (5). In an autopsy series of 39 patients dying from COVID-19, the virus was not detectable in the myocardium in 38% of patients, whereas 31% had a high viral load above 1000 copies in the heart (6). Accordingly, SARS-CoV-2 infection can damage the heart both directly and indirectly (see the figure). SARS-CoV-2 exhibited a striking ability to infect cardiomyocytes derived from induced pluripotent stem cells (iPSCs) in vitro, leading to a distinctive pattern of heart muscle cell fragmentation, with “complete dissolution of the contractile machinery” (7). Some of these findings were verified from patient autopsy specimens. In another iPSC study, SARS-CoV-2 infection led to apoptosis and cessation of beating within 72 hours of exposure (8). Besides directly infecting heart muscle cells, viral entry has been documented in the endothelial cells that line the blood vessels to the heart and multiple vascular beds. A secondary immune response to the infected heart and endothelial cells (endothelitis) is just one dimension of many potential indirect effects. These include dysregulation of the renin-angiotensin-aldosterone system that modulates blood pressure, and activation of a proinflammatory response involving platelets, neutrophils, macrophages, and lymphocytes, with release of cytokines and a prothrombotic state. A propensity for clotting, both in the microvasculature and large vessels, has been reported in multiple autopsy series and in young COVID-19 patients with strokes... Published in Science (Sept. 23, 2020): https://doi.org/10.1126/science.abe2813
|



 Your new post is loading...
Your new post is loading...