Your new post is loading...

|
Scooped by
Juan Lama
|
Ancient genomes from the herpes virus that commonly causes lip sores – and currently infects some 3.7 billion people globally – have been uncovered and sequenced for the first time by an international team of scientists led by the University of Cambridge. Latest research suggests that the HSV-1 virus strain behind facial herpes as we know it today arose around five thousand years ago, in the wake of vast Bronze Age migrations into Europe from the Steppe grasslands of Eurasia, and associated population booms that drove rates of transmission. Herpes has a history stretching back millions of years, and forms of the virus infect species from bats to coral. Despite its contemporary prevalence among humans, however, scientists say that ancient examples of HSV-1 were surprisingly hard to find. The authors of the study, published in the journal Science Advances, say the Neolithic flourishing of facial herpes detected in the ancient DNA may have coincided with the advent of a new cultural practice imported from the east: romantic and sexual kissing. “The world has watched COVID-19 mutate at a rapid rate over weeks and months. A virus like herpes evolves on a far grander timescale,” said co-senior author Dr Charlotte Houldcroft, from Cambridge’s Department of Genetics. “Facial herpes hides in its host for life and only transmits through oral contact, so mutations occur slowly over centuries and millennia. We need to do deep time investigations to understand how DNA viruses like this evolve,” she said. “Previously, genetic data for herpes only went back to 1925.” The team managed to hunt down herpes in the remains of four individuals stretching over a thousand-year period, and extract viral DNA from the roots of teeth. Herpes often flares up with mouth infections: at least two of the ancient cadavers had gum disease and a third smoked tobacco. The oldest sample came from an adult male excavated in Russia’s Ural Mountain region, dating from the late Iron Age around 1,500 years ago. Two further samples were local to Cambridge, UK. One a female from an early Anglo-Saxon cemetery a few miles south of the city, dating from 6-7th centuries AD. The other a young adult male from the late 14th century, buried in the grounds of medieval Cambridge’s charitable hospital (later to become St. John’s College), who had suffered appalling dental abscesses. The final sample came from a young adult male excavated in Holland: a fervent clay pipe smoker, most likely massacred by a French attack on his village by the banks of the Rhine in 1672. “We screened ancient DNA samples from around 3,000 archaeological finds and got just four herpes hits,” said co-lead author Dr Meriam Guellil, from Tartu University’s Institute of Genomics. “By comparing ancient DNA with herpes samples from the 20th century, we were able to analyse the differences and estimate a mutation rate, and consequently a timeline for virus evolution,” said co-lead author Dr Lucy van Dorp, from the UCL Genetics Institute. Co-senior author Dr Christiana Scheib, Research Fellow at St. John’s College, University of Cambridge, and Head of the Ancient DNA lab at Tartu University, said: “Every primate species has a form of herpes, so we assume it has been with us since our own species left Africa.” “However, something happened around five thousand years ago that allowed one strain of herpes to overtake all others, possibly an increase in transmissions, which could have been linked to kissing.” The researchers point out that the earliest known record of kissing is a Bronze Age manuscript from South Asia, and suggest the custom – far from universal in human cultures – may have travelled westward with migrations into Europe from Eurasia. In fact, centuries later, the Roman Emperor Tiberius tried to ban kissing at official functions to prevent disease spread, a decree that may have been herpes-related. However, for most of human prehistory, HSV-1 transmission would have been “vertical”: the same strain passing from infected mother to newborn child. Two-thirds of the global population under the age of 50 now carry HSV-1, according to the World Health Organisation. For most of us, the occasional lip sores that result are embarrassing and uncomfortable, but in combination with other ailments – sepsis or even COVID-19, for example – the virus can be fatal. In 2018, two women died of HSV-1 infection in the UK following Caesarean births. “Only genetic samples that are hundreds or even thousands of years old will allow us to understand how DNA viruses such as herpes and monkeypox, as well as our own immune systems, are adapting in response to each other,” said Houldcroft. The team would like to trace this hardy primordial disease even deeper through time, to investigate its infection of early hominins. “Neanderthal herpes is my next mountain to climb,” added Scheib. Publisjhed in Science Advances (July 27, 2022): https://doi.org/10.1126/sciadv.abo4435

|
Scooped by
Juan Lama
|
Three cell-based models shed light on how herpes simplex virus type 1 (HSV-1) infection, which can spread to the fetal brain during pregnancy, may contribute to various neurodevelopmental disabilities and long-term neurological problems into adulthood, according to a study published October 22, 2020 in the open-access journal PLOS Pathogens by Pu Chen and Ying Wu of Wuhan University, and colleagues. HSV-1 is a highly prevalent pathogen that can cause lifelong neurological problems such as cognitive dysfunction, learning disabilities, and dementia. But progress in understanding the role of HSV-1 in human fetal brain development has been hampered by restricted access to fetal human brain tissue as well as limitations of existing animal models. To address this gap in knowledge, the researchers generated three different cell-based neurodevelopmental disorder models, including a 2-D layer of cells and a 3-D brain-like structure. These models are based on human induced pluripotent stem cells (hiPSCs) - immature, embryonic stem cell-like cells that are generated by genetically reprogramming specialized adult cells. HSV-1 infection in neural stem cells derived from hiPSCs resulted in activation of the caspase-3 apoptotic pathway, which initiates programmed cell death. HSV-1 infection also impaired the production of new neurons, and hindered the ability of hiPSC-derived neural stem cells to convert into mature neurons through a process called neuronal differentiation. Moreover, the HSV-1-infected brain organoids mimicked the pathological features of neurodevelopmental disorders in the human fetal brain, including impaired neuronal differentiation and abnormalities in brain structure. In addition, the 3-D model showed that HSV-1 infection promotes the abnormal proliferation and activation of non-neuronal cells called microglia, accompanied by the activation of inflammatory molecules, such as TNF-α, IL-6, IL-10, and IL-4. According to the authors, the findings open new therapeutic avenues for targeting viral reservoirs relevant to neurodevelopmental disorders. The authors add, "This study provides novel evidence that HSV-1 infection impaired human brain development and contributed to the neurodevelopmental disorder pathogen hypothesis". Original study published in PLOS Pathogens (Oct. 22): https://doi.org/10.1371/journal.ppat.1008899

|
Scooped by
Juan Lama
|
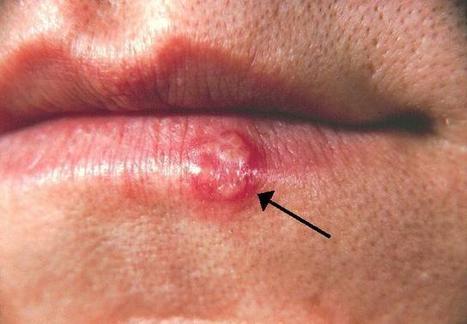
Oral herpes is a highly prevalent infection caused by herpes simplex virus 1 (HSV-1). After an initial infection of the oral cavity, HSV-1 remains latent in sensory neurons of the trigeminal ganglia. Episodic reactivation of the virus leads to the formation of mucocutaneous lesions (cold sores), but asymptomatic reactivation accompanied by viral shedding is more frequent and allows virus spread to new hosts. HSV-1 DNA has been detected in many oral tissues. In particular, HSV-1 can be found in periodontal lesions and several studies associated its presence with more severe periodontitis pathologies. Since gingival fibroblasts may become exposed to salivary components in periodontitis lesions, we analyzed the effect of saliva on HSV-1 and -2 infection of these cells. We observed that human gingival fibroblasts can be infected by HSV-1. However, pre-treatment of these cells with saliva extracts from some but not all individuals led to an increased susceptibility to infection. Furthermore, the active saliva could expand HSV-1 tropism to cells that are normally resistant to infection due to the absence of HSV entry receptors. The active factor in saliva was partially purified and comprised high molecular weight complexes of glycoproteins that included secretory Immunoglobulin A. Interestingly, we observed a broad variation in the activity of saliva between donors suggesting that this activity is selectively present in the population. The active saliva factor, has not been isolated, but may lead to the identification of a relevant biomarker for susceptibility to oral herpes. The presence of a salivary factor that enhances HSV-1 infection may influence the risk of oral herpes and/or the severity of associated oral pathologies. Published on PLOS One on October 3, 2019: https://doi.org/10.1371/journal.pone.0223299

|
Scooped by
Juan Lama
|
Just two days after announcing its R&D chief was stepping down, Gilead Sciences has announced it will be buying up a few of Novartis’ unwanted early-stage infection assets. The West Coast Big Biotech, which has been shuffling its execs over the past few weeks, today licensed three preclinical antiviral programs from Novartis including investigational agents with the potential to treat human rhinovirus, influenza and herpes. For its part, Novartis gets an undisclosed upfront payment, with biobucks worth $291 million as well as royalties on annual net sales. “Today’s announcement builds on Gilead’s heritage in antiviral research and development. We look forward to applying this expertise to advance the development of potential new treatments for viruses with limited therapeutic options,” said John McHutchison, M.D., Gilead’s outgoing chief scientific officer and head of R&D. Gilead has been pivoting toward cancer in recent years, most notably though its $12 billion buyout of Kite Pharma and its CAR-T drugs, but anti-infectives against hepatitis C and HIV remain the base of the company and seemingly still an important part of its future.
|

|
Scooped by
Juan Lama
|
Infections with the Epstein-Barr virus (EBV), the most common member of the herpes virus family, may contribute to the development of Sjögren’s syndrome by increasing the proportion of immune cells with autoimmune functions, a recent study suggests. Therapies targeting the interaction between EBV and activated immune cells may be useful in the clinic, the scientists said. The study, “Association between EBV serological patterns and lymphocytic profile of SjS patients support a virally triggered autoimmune epithelitis,” was published in the journal Nature, Scientific Reports. Sjögren’s syndrome, which predominantly affects middle-aged women, is characterized by mistaken immune attacks on the tear and salivary glands by cells known as lymphocytes, leading to disease symptoms. The two main types of lymphocytes, B-cells and T-cells, are normally involved in fighting infections from foreign pathogens such as bacteria and parasites. In Sjögren’s, however, evidence suggests that an infection may cause T-cells to enter the salivary and tear glands and support the disease-causing hyperactivity of B-cells, which are responsible for generating self-antibodies. Recently, follicular helper T-cells (Tfh) have been suggested to play a role in Sjögren’s development because they are found in higher levels in the bloodstream of Sjögren’s patients and are a major source of the immune signaling protein interleukin (IL)-21, which regulates B-cell survival. Furthermore, Tfh cells can express a receptor called CXCR5, which induces their migration toward B-cell sites. This receptor also has been found on a subset of cytotoxic (CD8+) T-cells thought to be involved in Sjögren’s by regulating B-cell responses and abnormal antibody production. While the underlying causes of Sjögren’s are still unclear, infection with EBV is a candidate for triggering the disease. Nevertheless, the impact of EBV infection on the B-cell profile found in Sjögren’s patients needs further examination. Now, a team of scientists based at the Comprehensive Health Research Centre, in Portugal, investigated subsets of B- and T-cells circulating in the bloodstream of Sjögren’s patients and assessed their relation to EBV infection. The study involved 57 people with Sjögren’s, of which 98.2% were women, with a median age of 60.6 years, and a median disease duration of 11.3 years. A group of 20 people with the autoimmune disorder rheumatoid arthritis (RA) and 24 healthy people (controls) also were enrolled for comparison. Compared with the healthy controls, the Sjögren’s patients had fewer helper T-cell numbers and percentages. The number of Tfh cells expressing CXCR5 was lower in Sjögren’s than in controls and RA patients. In contrast, the percentage of helper T-cells secreting IL-21 significantly increased in Sjögren’s participants compared with both RA patients and controls. Moreover, there was a significant correlation between higher IL-21-secreting T-cells and more Tfh cells with CXCR5. Cytotoxic T-cell percentages were significantly elevated in Sjögren’s compared with controls, but not in overall numbers. In comparison with both controls and RA patients, cytotoxic T-cells secreting IL-21 were increased as well. Higher levels of cytotoxic T-cells that express CXCR5 correlated with more disease activity, as assessed with the EULAR Sjögren’s disease activity index (ESSDAI), “suggesting their relevant role” in disease development, the team wrote. The percentages of non-activated B-cells (naïve) were significantly higher in Sjögren’s patients when compared with healthy controls and RA patients, and also presented higher absolute counts compared with RA, as “previously reported in the literature,” the team added. Next, the researchers divided patients into three subgroups according to their EBV profile, including those with previous infection, recent infection and EBV reactivation, or no evidence of active infection. Sjögren’s participants with recent infection or EBV reactivation had earlier disease onset and shorter disease duration. A majority of patients with a previous infection and/or a recent infection/reactivation had active disease at the time of recruitment, but patients with a previous infection showed higher disease activity scores. In contrast, the lowest disease activity scores were found in patients without active EBV infection. Regarding T-cells, patients with a previous infection and recent infection/activation had increased Tfh cells expressing CXCR5 as compared with those without active infection. The subset of B-cells that make antibodies also was increased in patients with a previous infection or recent infection/activation, compared with those without active EBV infection. “Our results suggest EBV-infection contributes to B- and T-cell differentiation towards the effector [cells] typical of [Sjögren’s syndrome],” the investigators wrote. “Considering the possible pathogenic role of EBV in the [development] of [Sjögren’s syndrome], therapies directed towards the interaction between EBV and activated effector T-cells and B-cells could halt the EBV-triggered lymphocytic activation, and have a relevant clinical applicability,” they added. Findings publshed in Nature Reports (Feb 18, 2021): https://doi.org/10.1038/s41598-021-83550-0

|
Scooped by
Juan Lama
|
Infectious disease researchers have used a gene editing approach to remove latent herpes simplex virus 1, or HSV-1, also known as oral herpes. In animal models, the findings show at least a 90 percent decrease in the latent virus, enough researchers expect that it will keep the infection from coming back. The study, published August 18 in Nature Communications, used two sets of genetic scissors to damage the virus's DNA, fine-tuned the delivery vehicle to the infected cells, and targeted the nerve pathways that connect the neck with the face and reach the tissue where the virus lies dormant in individuals with the infection. "This is the first time that scientists have been able to go in and actually eliminate most of the herpes in a body," said senior author Dr. Keith Jerome, professor in the Vaccine and Infectious Disease Division at Fred Hutch. "We are targeting the root cause of the infection: the infected cells where the virus lies dormant and are the seeds that give rise to repeat infections." Most research on herpes has focused on suppressing the recurrence of painful symptoms, and Jerome said that his team is taking a completely different approach by focusing on how to cure the disease. "The big jump here is from doing this in test tubes to doing this in an animal," said Jerome, who also leads the Virology Division at UW Medicine. "I hope this study changes the dialog around herpes research and opens up the idea that we can start thinking about cure, rather than just control of the virus." Two-thirds of the world population under the age of 50 have HSV-1, according to the World Health Organization. The infection primarily causes cold sores and is lifelong. In the study, the researchers used two types of genetic scissors to cut the DNA of the herpes virus. They found that when using just one pair of the scissors the virus DNA can be repaired in the infected cell. But by combining two scissors -- two sets of gene-cutting proteins called meganucleases that zero in on and cut a segment of herpes DNA -- the virus fell apart. "We use a dual meganuclease that targets two sites on the virus DNA," said first author Martine Aubert, a senior staff scientist at Fred Hutch. "When there are two cuts, the cells seem to say that the virus DNA is too damaged to be repaired and other molecular players come in to remove it from the cell body." The dual genetic scissors are introduced into the target cells by delivering the gene coding for the gene-cutting proteins with a vector, which is a harmless deactivated virus that can slip into infected cells. The researchers injected the delivery vector into a mouse model of HSV-1 infection, and it finds its way to the target cells after entering the nerve pathways. The researchers found a 92% reduction in the virus DNA present in the superior cervical ganglia, the nerve tissue where the virus lies dormant. The reductions remained for at least a month after the treatment and is enough the researchers say to keep the virus from reactivating. The team did other comparisons to fine-tune the gene editing approach: - Gene cuts with meganucleases were more efficient that with CRISPR/Cas9. - Refining the vector delivery mechanism, they found the adeno-associated virus (AAV) vector that was the most efficient at getting the gene edits to cells infected with the virus. The researchers are pursuing a similar strategy for herpes simplex 2, which causes genital herpes. They expect it to take at least 3 years to move toward clinical trials. "This is a curative approach for both oral and genital HSV infection," Aubert said. "I see it going into clinical trials in the near future." Original study published in NAt. Medicine (August 18, 2020): https://doi.org/10.1038/s41467-020-17936-5

|
Scooped by
Juan Lama
|
Latent herpes virus reactivation has been documented in more than half of astronauts during space shuttle and International Space Station missions, and according to a recent study funded by NASA, the cause is stress.“Herpes virus is a broad category of viruses, beyond the small subset that cause sexually transmitted diseases.Most humans become infected early in life with one or more, and never fully clear these viruses,” Satish Mehta, PhD, senior scientist in the immunology/virology lab at the Johnson Space Center in Houston, told Infectious Disease News. Results showed that 47 out of 89 (53%) astronauts on short space shuttle flights, and 14 out of 23 (61%) on longer space station missions shed herpes viruses in their saliva or urine samples. According to the study, astronauts shed four of the eight major human herpes viruses: Epstein-Barr, varicella-zoster and herpes simplex-1 in saliva and cytomegalovirus in urine. The researchers said most astronauts were asymptomatic, with only six developing symptoms. “Larger quantities and increased frequencies for these viruses were found during spaceflight as compared to before or after flight samples and their matched healthy controls,” Mehta and colleagues wrote. Mehta explained why: “[The] short answer is stress. In people with reduced immunity, such as the elderly or stressed individuals, these viruses can awaken and cause disease,” he said. “Although NASA believes there is no clinical risk to astronauts during orbital spaceflight, there is concern that during deep space exploration missions, there may be clinical risks related to viral shedding. Although we do not have a serious clinical problem related to herpes viruses, their reactivation is an excellent ‘flag,’ or biomarker, for stress and reduced immunity.” In the study, Mehta and colleagues noted that continued viral shedding after a flight can pose a potential risk for crew who may encounter infants, seronegative adults or immunocompromised people, so protocols have been put in place. The study was published in February 2019 in Frontiers in Microbiology - Virology: https://doi.org/10.3389/fmicb.2019.00016
|



 Your new post is loading...
Your new post is loading...