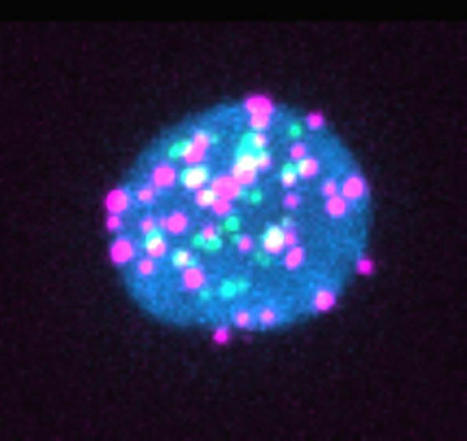
Dormant HIV actively produces RNA and proteins during anti-retroviral therapy. Patients with HIV usually carry a reservoir of HIV-infected cells that can re-emerge if anti-retroviral therapy stops. These reservoirs have long been thought to be dormant, but two independent groups of researchers report that a subset of these cells spontaneously produce HIV RNA and proteins that may impact patients’ HIV-specific immune responses. Their findings are published in the journal Cell Host & Microbe in the articles titled: “Spontaneous HIV expression during suppressive ART is associated with the magnitude and function of HIV-specific CD4+ and CD8+ T cells” and “An active HIV reservoir during ART is associated with maintenance of HIV-specific CD8+ T cell magnitude and short-lived differentiation status.” “It’s a deceptively dormant virus,” said immunovirologist Daniel Kaufmann, MD, of the University Hospital of Lausanne, the University of Lausanne, and l’Université de Montréal, and who is senior author on one of the papers. “Even in people who are treated, HIV continues to have some activity, and it continues to interact with the immune system. We have to understand if these ongoing interactions have clinically relevant consequences.”
Previous studies have shown that when “dormant” HIV reservoir cells are reactivated in the lab, they produce viral RNA and proteins, but it wasn’t clear whether this phenomenon was happening inside the bodies of people with HIV. “We wanted to understand if this phenomenon is real and, if it is, what parts of the virus are being expressed and do they have an impact on the immune system,” explained Kaufmann. The researchers took blood samples from 18 people with HIV who had all been on antiretrovirals for more than three years. Then, they used a lab method called RNA flow cytometry to sort CD4+ or “helper” T cells based on whether they were infected with HIV and, then further, whether they were actively producing HIV RNA or proteins. The researchers also characterized the T cells by role—e.g., whether they were the type of T helper cell that combats intracellular viruses or the type that combats extracellular bacteria—to determine whether any subtypes of CD4+ T cells were more likely to host HIV reservoirs. “Our technique allows us to look at the individual cells to see if they contain the virus and which parts of the virus they are expressing,” said Mathieu Dubé, an immunovirologist at l’Université de Montréal and first author on the paper led by Kaufmann. “For each patient, we could estimate how many of these cells are still active, and we could also look for associations between viral characteristics and cell characteristics.” The researchers observed that 14 of the 18 patients had HIV reservoirs that spontaneously produced viral RNA. For seven of the 18 patients, the viral reservoirs also produced viral proteins including p24, a component of HIV’s shell. “Most of the virus that remains in the body is defective or junk virus that cannot really multiply, but we found these defective viruses can still produce virus RNA and sometimes proteins,” said Kaufmann.
“Our data suggest that the RNA and proteins produced by these viral reservoirs could be drivers of inflammation,” said Kaufmann. “This could be important because a subset of the people who are successfully treated with anti-retroviral therapy for HIV still have negative consequences of living with the infection—for example, accelerated cardiac disease, frailty, and premature osteoporosis.” The second paper, led by immunologist Lydie Trautmann, PhD, at the Vaccine and Gene Therapy Institute of Oregon Health and Science University, also reported that a subset of CD4+ T cells spontaneously express viral RNA during anti-retroviral therapy. “Our study suggests that residual immune dysfunction driven by the active HIV reservoir on anti-retroviral therapies could contribute to the lack of viral control after analytical treatment interruption by preventing the differentiation of functional stem-like self-renewing HIV-specific CD8+ T cells that can mount efficient rapid recall responses,” the authors wrote. “Therefore, HIV remission strategies will likely need to target transcriptionally active proviruses producing viral proteins during anti-retroviral therapies to harness HIV-specific CD8+ T cells to control rebounding of the virus after therapy cessation.”
Research cited published in Cell and Host Microbe (September 2023):



 Your new post is loading...
Your new post is loading...