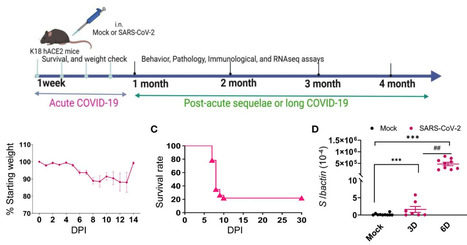
Viral variant is one known risk factor associated with post-acute sequelae of COVID-19 (PASC), yet the pathogenesis is largely unknown. Here, we studied SARS-CoV-2 Delta variant-induced PASC in K18-hACE2 mice. The virus replicated productively, induced robust inflammatory responses in lung and brain tissues, and caused weight loss and mortality during the acute infection. Longitudinal behavior studies in surviving mice up to 4 months post-acute infection revealed persistent abnormalities in neuropsychiatric state and motor behaviors, while reflex and sensory functions recovered over time. In the brain, no detectable viral RNA and minimal residential immune cell activation was observed in the surviving mice post-acute infection.
Transcriptome analysis revealed persistent activation of immune pathways, including humoral responses, complement, and phagocytosis, and gene expression levels associated with ataxia telangiectasia, impaired cognitive function and memory recall, and neuronal dysfunction and degeneration. Furthermore, surviving mice maintained potent systemic T helper 1 prone cellular immune responses and strong sera neutralizing antibodies against Delta and Omicron variants months post-acute infection. Overall, our findings suggest that infection in K18-hACE2 mice recapitulates the persistent clinical symptoms reported in long-COVID patients and provides new insights into the role of systemic and brain residential immune factors in PASC pathogenesis.
Published in Frontiers in Immunology (May 2, 2024):



 Your new post is loading...
Your new post is loading...