Your new post is loading...

|
Scooped by
Juan Lama
|
The FDA has issued an emergency use authorization for tocilizumab to treat hospitalized patients receiving corticosteroids who require supplemental oxygen, mechanical ventilation or extracorporeal membrane oxygenation, according to a press release. The emergency use authorization for tocilizumab (Actemra, Genentech) — now the fourth monoclonal antibody authorized for COVID-19 — is is specifically for hospitalized adults and children aged 2 years and older; it is not intended for outpatients or as a treatment for COVID-19, the FDA noted. “Today’s action demonstrates the FDA’s commitment to making new therapies available through every stage of the global COVID-19 pandemic,” Patrizia Cavazzoni, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release. “Although vaccines have been successful in decreasing the number of patients with COVID-19 who require hospitalization, providing additional therapies for those who do become hospitalized is an important step in combating this pandemic.” The FDA based its emergency use authorization on results from four randomized, controlled trials — RECOVERY, EMPACTA, COVACTA and REMDACTA — that evaluated the safety and efficacy of tocilizumab in more than 5,500 hospitalized patients with COVID-19. In particular, the RECOVERY and EMPACTA trials provided the “most important scientific evidence on the potential benefit of Actemra for its authorized use,” the FDA noted. In the RECOVERY trial, hospitalized patients with severe COVID-19 pneumonia (n=4,116) were randomized to receive either tocilizumab plus standard care (n=2,022) or standard care alone (n=2,094). The primary endpoint was death through 28 days of follow-up. According to the FDA, the “results of the primary analysis were statistically significant” with the probability of death by day 28 estimated to be 30.7% for patients receiving tocilizumab and 34.9% for patients receiving standard care alone. Additionally, the median time to hospital discharge was 19 days in the tocilizumab group compared with more than 28 days in the standard care group. Supplemental data from the EMPACTA trial, in which hospitalized patients with COVID-19 pneumonia (n=389) were randomized to receive tocilizumab (n=249) or standard care (n=128), demonstrated a lower risk for progression to mechanical ventilation or death by day 28 in hospitalized patients who received tocilizumab (12% vs. 19.3%). “Even with the availability of vaccines and declines in deaths from COVID-19 in various parts of the world, we continue to see new hospitalizations from severe forms of the disease,” Levi Garraway, MD, PhD, chief medical officer and head of Global Product Development at Genentech, said in a company press release. “We are pleased that Actemra is now authorized as an option that may help improve outcomes for adults and children hospitalized with COVID-19 in the United States.” The emergency use authorization requires that fact sheets be made available to health care providers and patients, parents and caregivers featuring information on the potential side effects, which include constipation, anxiety, diarrhea, insomnia, hypertension and nausea. See also FDA information (June 24, 2021): https://www.fda.gov/media/150345/download

|
Scooped by
Juan Lama
|
This use of adaptive clinical trials is letting researchers work faster and collaborate better to find solutions for the Covid-19 pandemic. Viruses like SARS-CoV-2 can circle the globe with astonishing speed by taking advantage of human networks. The global medical research community couldn’t immediately contain it because it had no comparable network for defense — but we are moving quickly to create one. Employing adaptive clinical trials will help. The initial response to Covid-19, driven by the best intentions, was to stand up more than 1,000 clinical studies. Many were small, uncontrolled, and unlikely to yield reliable information. This is one reason vaccines and definitive cures continue to elude us. Even so, researchers have scored some remarkable wins. Less than a month after the first Covid-19 cases surfaced in late December, a research team sequenced the virus. Since then, other teams have identified cellular proteins that bind to it, shared data in open-access journals, and kicked off at least two dozen credible Phase 3 trials for vaccines, antivirals, and other treatments. More milestones will follow as we expand research networks and exploit major innovations in clinical trial design. One advance in particular — an iterative, long-duration study known as an adaptive platform trial — is helping researchers up their game. The use of adaptive clinical trials allows them to simultaneously test multiple interventions against a single, shared control arm, add and eliminate treatments as the trial progresses, and update the study design as the treatment landscape changes. In contrast, each traditional standalone Phase 3 trial must recruit patients to its own control group and test just one hypothesis. Even designing in techniques such as subset analysis and adaptation, these are extravagant undertakings that can’t match the supple efficiency and productivity of well-managed adaptive platform studies. The adaptive clinical trials approach has already led to a series of high-value, actionable research results: - In April, data from an adaptive platform study called the Adaptive Covid-19 Treatment Trial, or ACTT, from the National Institutes of Health, validated the clinical benefits of remdesivir, an antiviral agent, in patients hospitalized with Covid-19.
- A few weeks later, the similarly-designed Randomised Evaluation of Covid-19 Therapy (RECOVERY) trial in the United Kingdom demonstrated the benefits of dexamethasone, a widely used steroid.
- RECOVERY further showed that the malaria drug hydroxychloroquine provides little help for people with Covid-19, an invaluable contribution in the face of public confusion and pressure to use the medicine. It also showed that a combination of HIV treatments, lopinavir and ritonavir, was ineffective in treating the disease....
Imagine how the Covid-19 picture might look today if initiatives like these had been staged in advance — anticipating an inevitable pandemic — and networked into a globe-spanning adaptive platform ecosystem. Such an uber-program would allow researchers on every continent to pool knowledge, data, analytics, materials, and other resources, and adding and deleting trial arms based on interim rea..douts, real-world data, and insights from the field. Adaptive platform trials are not new. The methodology was already gaining traction in oncology and other areas before Covid-19 struck. They’re a subset of master protocol trials that Janet Woodcock, the Food and Drug Administration’s long-serving director for drug evaluation and research, and Lisa LaVange explored in a review article in the New England Journal of Medicine in 2017. This May, the FDA’s updated guidance on Covid-19 drug development recognized the potential of these trial designs, and the agency is likely to issue further recommendations....

|
Scooped by
Juan Lama
|
Aims: Studies have indicated that chloroquine (CQ) shows antagonism against COVID-19 in vitro. However, evidence regarding its effects in patients is limited. This study aims to evaluate the efficacy of hydroxychloroquine (HCQ) in the treatment of patients with COVID-19. Main methods: From February 4 to February 28, 2020, 62 patients suffering from COVID-19 were diagnosed and admitted to Renmin Hospital of Wuhan University. All participants were randomized in a parallel-group trial, 31 patients were assigned to receive an additional 5-day HCQ (400 mg/d) treatment, Time to clinical recovery (TTCR), clinical characteristics, and radiological results were assessed at baseline and 5 days after treatment to evaluate the effect of HCQ. Key findings: For the 62 COVID-19 patients, 46.8% (29 of 62) were male and 53.2% (33 of 62) were female, the mean age was 44.7 (15.3) years. No difference in the age and sex distribution between the control group and the HCQ group. But for TTCR, the body temperature recovery time and the cough remission time were significantly shortened in the HCQ treatment group. Besides, a larger proportion of patients with improved pneumonia in the HCQ treatment group (80.6%, 25 of 31) compared with the control group (54.8%, 17 of 31). Notably, all 4 patients progressed to severe illness that occurred in the control group. However, there were 2 patients with mild adverse reactions in the HCQ treatment group. Significance: Among patients with COVID-19, the use of HCQ could significantly shorten TTCR and promote the absorption of pneumonia. Preprint available at medRxiv since March 31, 2020: https://doi.org/10.1101/2020.03.22.20040758
|

|
Scooped by
Juan Lama
|
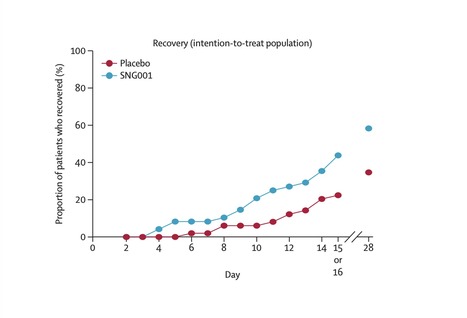
Background Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection carries a substantial risk of severe and prolonged illness; treatment options are currently limited. We assessed the efficacy and safety of inhaled nebulised interferon beta-1a (SNG001) for the treatment of patients admitted to hospital with COVID-19. Methods We did a randomised, double-blind, placebo-controlled, phase 2 pilot trial at nine UK sites. Adults aged 18 years or older and admitted to hospital with COVID-19 symptoms, with a positive RT-PCR or point-of-care test, or both, were randomly assigned (1:1) to receive SNG001 (6 MIU) or placebo by inhalation via a mouthpiece daily for 14 days. The primary outcome was the change in clinical condition on the WHO Ordinal Scale for Clinical Improvement (OSCI) during the dosing period in the intention-to-treat population (all randomised patients who received at least one dose of the study drug). The OSCI is a 9-point scale, where 0 corresponds to no infection and 8 corresponds to death. Multiple analyses were done to identify the most suitable statistical method for future clinical trials. Safety was assessed by monitoring adverse events for 28 days. This trial is registered with Clinicaltrialsregister.eu (2020-001023-14) and ClinicalTrials.gov (NCT04385095); the pilot trial of inpatients with COVID-19 is now completed. Findings Between March 30 and May 30, 2020, 101 patients were randomly assigned to SNG001 (n=50) or placebo (n=51). 48 received SNG001 and 50 received placebo and were included in the intention-to-treat population. 66 (67%) patients required oxygen supplementation at baseline: 29 in the placebo group and 37 in the SNG001 group. Patients receiving SNG001 had greater odds of improvement on the OSCI scale (odds ratio 2·32 [95% CI 1·07–5·04]; p=0·033) on day 15 or 16 and were more likely than those receiving placebo to recover to an OSCI score of 1 (no limitation of activities) during treatment (hazard ratio 2·19 [95% CI 1·03–4·69]; p=0·043). SNG001 was well tolerated. The most frequently reported treatment-emergent adverse event was headache (seven [15%] patients in the SNG001 group and five [10%] in the placebo group). There were three deaths in the placebo group and none in the SNG001 group. Interpretation Patients who received SNG001 had greater odds of improvement and recovered more rapidly from SARS-CoV-2 infection than patients who received placebo, providing a strong rationale for further trials. Published in The Lancet Respiratory Medicine (Nov. 12, 2020):

|
Scooped by
Juan Lama
|
Dexamethasone, a readily available steroids, reduced deaths by a third in patients hospitalized with Covid-19 in a large study, researchers announced. In a statement, Patrick Vallance, the U.K. government’s chief scientific adviser, called the result “tremendous news” and “a ground-breaking development in our fight against the disease.” Scott Gottlieb, a former commissioner of the U.S. Food and Drug Administration, called it “a very positive finding” in an interview on CNBC. “I think it needs to be validated, but it certainly suggests that this could be beneficial in this setting.” Atul Gawande, the surgeon, writer and public health researcher, urged caution, tweeting, “after all the retractions and walk backs, it is unacceptable to tout study results by press release without releasing the paper.” The study randomly assigned 2,104 patients to receive six milligrams of dexamethasone once a day, by mouth or intravenous injection. These were compared to 4,321 patients assigned to receive usual care alone. In patients who needed to be on a ventilator, dexamethasone reduced the death rate by 35%, meaning that doctors would prevent one death by treating eight ventilated patients. In those who needed oxygen but were not ventilated, the death rate was reduced 20%, meaning doctors would need to treat 25 patients to save one life. Both results were statistically significant. There was no benefit in patients who didn’t require any oxygen. The researchers running the study, called RECOVERY, decided to stop enrolling patients on dexamethasone on June 8 because they believed they had enough data to get a clear result. “Dexamethasone is the first drug to be shown to improve survival in COVID-19,” Peter Horby, one of the lead investigators of the study and a professor in the Nuffield Department of Medicine at the University of Oxford, said in a statement. He added that the drug should now become the standard treatment for patients with Covid-19 who need oxygen. “Dexamethasone is inexpensive, on the shelf, and can be used immediately to save lives worldwide.’”... See also: https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_final.pdf
|



 Your new post is loading...
Your new post is loading...