Your new post is loading...

|
Scooped by
Juan Lama
|
Nearly half of doses administered so far have gone to high-income countries — just 16 percent of the world’s population. The globe is quickly being split into coronavirus vaccine “haves” and “have-nots,” creating a gap that may define the next phase of the pandemic. Using publicly available figures from Our World in Data, The Washington Post found that nearly half — 48 percent — of all vaccine doses administered so far have gone to just 16 percent of the world’s population in what the World Bank considers high-income countries. Through the summer and fall of last year, wealthy nations cut deals directly with vaccine-makers, buying up a disproportionate share of early doses — and undermining a World Health Organization-backed effort, called Covax, to equitably distribute shots. So now, in a small number of relatively wealthy nations, including the United States, doses are relatively plentiful and mass immunization campaigns are progressing apace. But much of the world is still struggling to secure enough supply. For many, herd immunity is many months — if not years — away, which could extend the crisis. A team at Duke University’s Global Health Innovation Center found that high-income countries locked up 53 percent of near-term vaccine supply. They estimate that the world’s poorest 92 countries will not be able to reach a vaccination rate of 60 percent of their populations until 2023 or later. Israel has so far immunized the largest number of people per capita. As of April 19, nearly 60 percent of Israelis had received at least one dose and nearly 58 percent were fully vaccinated, according to Our World in Data. Though Israel was later than some countries to sign vaccine deals, it offered to pay premium prices and give drug companies access to its health-care data. The country reportedly spent $788 million on coronavirus vaccines by March, most notably on a large shipment of Pfizer-BioNTech’s RNA vaccine. While Israel has been criticized for neglecting the Palestinian population in its midst, its vaccination campaign has otherwise been deemed a success and has allowed the return of a more normal way of life, including the lifting of outdoor mask requirements. Britain is another country leading the way. Between developing, buying and administering vaccines, the country will spend about $16 billion, according to a National Audit Office estimate. To stretch supply as far as possible, Britain opted to space doses by several months, meaning that while nearly 50 percent of the country has had at least one shot, just over 16 percent is fully vaccinated. The campaign was set back this month when concerns about rare blood clots in people receiving the AstraZeneca vaccine led the government to restrict use in adults under 30. Still, early studies in Britain show significant reductions in infections and hospitalizations after a first dose of the AstraZeneca or Pfizer vaccines. And the country has been able to slowly begin lifting its lockdown. The United States, after experiencing one of the world’s most deadly outbreaks, is now the envy of the world with its abundant vaccine supply and rapidly progressing inoculation campaign. The country spent billions on vaccine development, deals and distribution. About 41 percent of U.S. residents have received at least one dose and more than 26 percent are fully vaccinated. As of this week, all Americans over the age of 18 are eligible to get a shot — a milestone that may renew questions about what the country plans to do with its predicted hundreds of millions of surplus doses. The Biden administration faces growing calls from public health advocates and activists to share, either by donating doses to countries in need, transferring technology to boost manufacturing capacity, or by easing export restrictions that have kept a disproportionate number of shots, as well as critical vaccine materials, in the United States.......

|
Scooped by
Juan Lama
|
Forget boosters and more trials. America’s overly prudent vaccination strategy is killing people. In the race to prevent ever more deaths from Covid-19, the United States faces two major problems: not having enough doses of vaccine on hand and struggling to deliver those that are available. According to data from the Centers for Disease Control and Prevention, as of Jan. 28 about 48.4 million doses had been distributed, but only 26.2 million administered. The Food and Drug Administration has granted emergency-use authorization to two vaccines requiring two doses — with the first shot and the booster to be delivered three weeks apart in the case of the Pfizer-BioNTech vaccine, and four weeks apart for the Moderna vaccine As of Thursday, just 4.2 million people in America had received both doses, according to the C.D.C. Uncertain about vaccine supplies, some inoculation centers have canceled first-time appointments while keeping second appointments on schedule. This is exactly backward. The Biden administration’s Covid-19 response and pandemic preparedness plan aims to provide 100 million doses within 100 days of the president’s inauguration, especially to people over 65, all essential workers (not only those in health care services) and “the highest-risk members of the public, including racial, ethnic and rural populations and those in congregate facilities.” Both the plan’s sense of urgency and its focus on some of the most vulnerable groups are welcome. But the proposal also backs the use of second doses and supports sticking to F.D.A.-recommended timelines for administering them, and that is misguided. We think more lives would be saved by providing as soon as possible (a) just one dose of vaccine (b) to all people who face the highest risk of dying from Covid-19, whatever the reason (advanced age, other medical conditions, severe obesity), and (c) just forgetting about any boosters for a while, maybe even a very long while. Second doses should be deferred for the time it takes to achieve this primary goal. The two vaccines’ manufacturers and the F.D.A. have been reluctant to endorse any change to the vaccination schedules that were tested in Phase 3 clinical trials, on grounds that other options weren’t tested and so their efficacy is unknown. On the face of it, this position seems sensible; yet under current circumstances, it is dangerously overcautious. The C.D.C. has authorized giving a second dose up to six weeks after the first one if sticking to the recommended interval is “not feasible.” But this is an arbitrary extension — it wasn’t tested any more than any other modification — and we think it’s too short to accomplish much good society-wide. Bear in mind that the clinical trials performed for both the Pfizer-BioNTech and Moderna vaccines weren’t designed to identify the optimal number of doses for inoculation, nor the perfect schedule for administering them. The goal of those trials was only to test whether two doses of vaccine given according to the schedule chosen by the manufacturers did confer sufficient protection against Covid-19. Note, too, that in the case of both those vaccines the intervals tested (three and four weeks) were unusually short. In standard schedules for childhood vaccines in the United States, for example, the gap between the first shot and the booster typically is no less than two months. The fact that the trials did not test whether administering a second dose much later, or not at all, might work just as well than the recommended schedule, or well enough, also means that it could work. And there is good reason to think that it would. With some vaccines, like those for hepatitis A or human papillomavirus (HPV), delaying the second dose by as long as several years has been shown to not decrease the protection conferred by the vaccine. In other cases, such as influenza vaccines, increasing the interval actually improves the response. (This likely is because the cells in the body that develop the immune memory necessary to repeatedly fend of infections can take weeks or months to develop.) In trials for another Covid-19 vaccine not currently available in the United States — this one developed by Oxford-AstraZeneca — study subjects were supposed to get a booster one month after the first shot but some received it up to 12 weeks later. Results for the latecomers were outside the trials’ formal ambit, but they are available, and they suggest that the subjects who waited to get their booster beyond the recommended delay displayed better immune responses than the subjects who got it on time. In non-pandemic situations, the usual approach is to give a vaccine to the wider public in precisely the same way that it was studied and vetted in Phase 3 trials, as well as to initiate new clinical trials or real-world studies to evaluate alternative ways of administering it. As a result of this practice, recommended doses were reduced for vaccines against, for example, HPV (from three to two) and meningococcal B in infants (from four to three) — with no significant reduction in immune responses. Such precedents may be one reason that some doctors and social scientists are now calling for promptly conducting studies to assess the performance of single doses of Pfizer-BioNTech’s and Moderna’s Covid-19 vaccines in young, healthy subjects, the group least likely to become severely sick with Covid-19. Yet this approach, too, misses the mark. With the pandemic raging as it is — one person is dying of Covid-19 about every 30 seconds in America — there is no time to wait for the results from additional clinical trials, which would take months to complete, before vaccine-rollout plans are modified. And studying the efficacy of single doses only among individuals who face a very low risk of dying from Covid-19 wouldn’t say much anyway about the viability of using single doses with the people most at risk of death.........

|
Scooped by
Juan Lama
|
The Trump administration moved on Tuesday to accelerate vaccinations of Americans against COVID-19, releasing the rest of the doses it had been keeping in reserve and recommending states immediately open inoculations to those aged 65 and over. Federal and state health officials have scrambled in recent days to step up vaccination programs that had given shots to only 9.3 million Americans as coronavirus infections remain at record highs in many U.S. states nearly two weeks into the new year. Many U.S. states had strict rules in place giving shots to healthcare workers and nursing home residents first, telling “non-essential workers” they might wait months for their turn. “We’ve already distributed more vaccine than we have healthcare workers and people in nursing homes,” U.S. Health and Human Services Secretary Alex Azar told ABC News. “We’ve got to get to more channels of administration.” Roughly 27.5 million doses have been distributed by the U.S. government to states so far, according to the U.S. Centers for Disease Control and Prevention.Azar said the outgoing administration, which had been keeping doses in reserve to make sure that all those who got a first inoculation receive their second shot on schedule, was now confident enough in the supply chain to release that stockpile. Last week, a spokesman for Joe Biden said the president-elect, who takes office on Jan. 20, would release more of the reserved doses. The pace of vaccinations has risen to 700,000 a day nationwide and was expected to hit 1 million a day within 10 days, officials said. “Michigan and states across the country remain ready to get more shots in arms, which is why the Trump Administration’s decision to grant our request and release millions of doses of the vaccine is so crucial,” Michigan Governor Gretchen Whitmer said in a statement. Whitmer, who had backed the lower vaccination age, is seeking permission from the U.S. government to purchase 100,000 vaccine doses directly from manufacturer Pfizer Inc. The U.S. Food and Drug Administration has authorized the vaccine from Pfizer and partner BioNTech SE and a second vaccine from Moderna Inc for emergency use. As of Monday night, the United States had reported a total of 22.5 million coronavirus infections and 376,188 deaths during the pandemic, the most of any country. Nearly 130,000 Americans were hospitalized with COVID-19 at midnight on Monday. GRIM SCENES AT CALIFORNIA HOSPITAL A Reuters tally has shown that the number of COVID-19 patients requiring hospitalization may have leveled off, at least temporarily, although public health officials warn that further spread may still be seen from holiday gatherings. California Health and Human Services Secretary Dr. Mark Ghaly cited several promising trends in COVID metrics statewide in recent days, including a slowing in confirmed daily case numbers and a leveling off in positive tests. The number of new COVID hospitalizations statewide has fallen to about 2,500 admissions a day over the past two days from a daily average of about 3,500 admissions in previous days. Ghaly called that “the biggest signal to me that things are beginning to flatten and potentially improve." Despite the encouraging statistics, staff at Providence St. Mary Medical Center in Apple Valley, California, said that the situation was grim. “Where in the beginning we were overloaded with a lot of patients - we still have a lot of patients - but now it seems like they’re sicker than they’ve ever been before,” said Mary Mendy, executive director of acute care services at the hospital some 90 miles northeast of Los Angeles. “And every day there’s Code Blues on the floor and more and more patients are updated to ICU. It’s devastating,” Mendy said. The latest surge has potentially been compounded by a more infectious variant of the virus first seen in the UK and now found in at least 10 U.S. states - California, Florida, New York, Colorado, Georgia, Indiana, Connecticut, Minnesota, Pennsylvania and Texas.

|
Scooped by
Juan Lama
|
LONDON (AP) — The U.K. on Monday became the first nation in the world to start using the COVID-19 vaccine developed by Oxford University and drugmaker AstraZeneca, ramping up a nationwide inoculation program as rising infection rates are putting an unprecedented strain on British hospitals. Brian Pinker, an 82-year-old dialysis patient, received the first shot at 7:30 a.m. at Oxford University Hospital. “The nurses, doctors and staff today have all been brilliant, and I can now really look forward to celebrating my 48th wedding anniversary with my wife, Shirley, later this year,” Pinker said in a statement released by the National Health Service. The rollout of the new vaccine comes at a crucial moment for U.K. authorities, who are battling a surge in infections blamed on a new virus variant that authorities have said is much more contagious. Scotland imposed a lockdown until the end of January amid increasing pressure on officials to tighten restrictions throughout the U.K. Prime Minister Boris Johnson, who has said tougher measures are imminent, announced a nationwide address at 8 p.m. The UK Parliament will be recalled to sit on Wednesday. “If you look at the numbers, there’s no question we will have to take tougher measures and we will be announcing those in due course,” Johnson said while visiting some of the people receiving the Oxford-AstraZeneca vaccine at Chase Farm Hospital in north London. The U.K. is in the midst of an acute outbreak, recording more than 50,000 new coronavirus infections a day over the past six days. On Sunday, it notched up another 54,990 cases and 454 more virus-related deaths to take its confirmed pandemic death toll total to over 75,000, one of the worst in Europe. Some areas northeast of London have infection rates of over 1,000 cases per 100,000 people. Scottish leader Nicola Sturgeon says that beginning Tuesday, people in Scotland will be legally required to stay at home except for essential reasons to help ease the pressure on hospitals and intensive care units. Under the new lockdown rules in Scotland, people can go out for exercise but can only meet one other person from another household. School closures are extended until February except for children of key workers and children under social care. “I am more concerned about the situation we face now than I have been at any time since March last year,” she said Scotland, which has its own devolved government, has often imposed stricter coronavirus restrictions than those in England throughout the pandemic. U.K. regulators last week authorized emergency use of the Oxford-AstraZeneca shot, giving public health officials a second vaccine in their medical arsenal. Britain’s mass vaccination program began Dec. 8 with the shot developed by New York-based Pfizer and its German partner BioNTech. Britain has secured the rights to 100 million doses of the Oxford-AstraZeneca vaccine, which is cheaper and easier to use than some of its rivals. In particular, it doesn’t require the super-cold storage needed for the Pfizer vaccine. The new vaccine will be administered at a small number of hospitals for the first few days so authorities can watch out for any adverse reactions. But the NHS said hundreds of new vaccination sites — including local doctors’ offices — will open later this week, joining the more than 700 vaccination sites already in operation. A “massive ramp-up operation” is now underway in the vaccination program, Johnson said. But aspects of Britain’s vaccination plans have spurred controversy. Both vaccines require two shots, and Pfizer had recommended that the second dose be given within 21 days of the first. But The U.K.’s Joint Committee on Vaccination and Immunization said authorities should give the first vaccine dose to as many people as possible, rather than setting aside shots to ensure others receive two doses. It has stretched out the time between the doses from 21 days to within 12 weeks. While two doses are required to fully protect against COVID-19, both provide high levels of protection after the first dose, the committee said. Making the first dose the priority will “maximize benefits from the vaccination program in the short term,” it said. Stephen Evans, a professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said policymakers are being forced to balance the potential risks of this change against the benefits in the middle of a deadly pandemic....
|

|
Scooped by
Juan Lama
|
Maccabi health fund releases preliminary results of a study comparing vaccinated and not vaccinated members’ likability to contract the disease and said vaccine 92% effective. A total of 371 out of 715,425 Israelis who passed at least a week after receiving two doses of the Pfizer coronavirus vaccine have contracted the virus – 0.04%, with 16 being sent to the hospital – according to a Health Ministry report released on Thursday. Immunity to COVID-19 is supposed to kick in a week after receiving the second dose of the Pfizer vaccine. According to the studies conducted by Pfizer, the vaccine had an efficacy of about 95%, which is considered very high. The Israeli data appear to confirm the inoculation’s effectiveness, showing an even more promising result. Later in the day, Maccabi Healthcare Services – one of the country’s four health maintenance organizations – released the first results of the vaccination campaign of its members, with the organization also comparing the data to a control group that did not get inoculated. Some 248,000 Maccabi members were already a week after the second shot as of Thursday. Of those, just 66 got infected with the virus, the majority of them over the age of 55 and about half of them with preexisting conditions. All those infected experienced only a mild form of the disease, and none were hospitalized. Over the same period of time, some 8,250 new cases of COVID-19 emerged in the control group of some 900,000 people having a diverse health profile. Those who were not inoculated were therefore 11 times more likely to get the disease than those who were immunized, showing 92% effectiveness. “The fact that seven to 18 days after receiving the second dose the vaccine shows a 92% efficacy is very encouraging data,” according to Dr. Anat Aka Zohar, head of Maccabi’s Information and Digital Health Division. “We will continue to monitor the situation to see if the number increases and reaches the 95% demonstrated during the Pfizer study.” Israel has established itself as a vaccination powerhouse. So far, more three million people have received their first dose of the Pfizer vaccine – including 82% of people over 60 – and 1.5 million have been given both shots. Beginning Thursday, Israel started vaccinating people as young as 35 at a pace of 200,000 shots per day.

|
Scooped by
Juan Lama
|
Israel is currently leading the global vaccination drive, with around 30 per cent of its citizens having had at least a single dose of a jab so far. - Israeli healthcare firm KSM Maccabi Research and Innovation Center on Friday reported on vaccination data
- It said there had been a 'significant decrease' coronavirus infections among vaccinated over-65s
- Also found that hospitalisations in the same group had plunged by more than 60 per cent
- Over 2.5 million of Israeli citizens have had the first vaccine dose, around 30 per cent of population
- Teenagers aged 16-18 are now being given the first dose of the vaccine, starting on Saturday
An Israeli healthcare group on Friday said coronavirus infections had plunged among people aged over 60 who had been vaccinated with the Pfizer Biontech vaccine. Israel is currently leading the global vaccination drive, with around 30 per cent of its citizens having had at least a single dose of a jab so far. But concern had risen globally over infection, death and hospitalisation rates in the country, which remained stubbornly high. Out of 82,930 active cases on Thursday, 1,918 were hospitalized. Last week, the hospitalisation figure was just over 1,000. Officials had hoped that the vaccine drive - which began on December 19 - would start to show an effect by mid-February But KSM Maccabi Research and Innovation Center claimed on Friday there had been a 'significant decrease' in the number of coronavirus infections among people aged over 60 who were vaccinated between December 19 and 24. After analysing data of more than 50,000 patients aged over 60, they also found that hospitalisations in the same group had plunged by more than 60 per cent. Israel secured access to large amounts of Pfizer's jab by agreeing to provide data about its citizens for the company to track how well the jab works. The new figures are a sign of hope that nationwide infections, deaths and hospitalisations could soon start to see a sustained fall. It came amid reports that England's chief medical officer was so infuriated by a newspaper story which claimed that a single dose of the Pfizer vaccine might only be 33 per cent effective that he threatened to report it to a press watchdog. Chris Whitty told colleagues The Guardian's report was 'total nonsense' which could threaten the uptake of the jab. KSM Maccabi Research and Innovation Center's report was based on data 50,777 members of Maccabi who were aged over 60 and were vaccinated 23 days ago. KSM, which is part of Israeli healthcare provider Maccabi, noted that there was a 'significant decrease within the vaccinated members aged 60+', reaching a decrease of around 60 per cent in new infections. They added that there was also a 'decrease of slightly more than 60 per cent in the number of new hospitalised patients.' However, KSM cautioned that 'on this level of efficiency, there should be no exemption from performing Corona tests, isolation, or the enablement of crowded gatherings, until additional convincing data is obtained. 'And of course continue to wear masks and keep social distancing, as recommended'. In their story about the effectiveness of a single dose of the Pfizer jab, The Guardian had quoted 'Israeli experts' as saying only a third of people who have received one injection were protected. No 10's vaccine advisers say the real figure is 89 per cent, starting 14 days after the first jab. It was reported yesterday that a single shot of the Pfizer vaccine had led to a 'major presence' of antibodies in 91 per cent of doctors and nurses who received it in Israel within 21 days. A source told the Mail On Sunday: 'It is not every day that a member of the liberal academic establishment is angered by The Guardian.' On Friday, Israel announced a further 6,159 new cases, an 18 per cent increase on the figure of 5,235 announced seven days ago, but down from Wednesdays and Thursdays totals, of 10,213 and 7,027 respectively. Since the rollout of vaccinations one month ago, more than 2.5 million of Israel's nine-million-strong population have been vaccinated already, the health ministry said on Friday. It came as the Israeli health ministry on Thursday announced it was allowing the inoculation of high school students aged 16-18, subject to parental approval. The health ministry had on Thursday announced it was allowing the inoculation of high school students aged 16-18, subject to parental approval. Expanding the campaign to include teens came days after Israel extended on Tuesday till the end of the month its third national coronavirus lockdown due to a surge in coronavirus infections despite the vaccinations.....

|
Scooped by
Juan Lama
|
The head of Operation Warp Speed says that by spreading out doses of the Moderna vaccine, more people would be able to get some level of protection sooner, a strategy opposed by other experts. Update (January 5): On Monday evening, the FDA stated that dosing in the US will occur as planned, based on available data regarding efficacy, saying: “We know that some of these discussions about changing the dosing schedule or dose are based on a belief that changing the dose or dosing schedule can help get more vaccine to the public faster. However, making such changes that are not supported by adequate scientific evidence may ultimately be counterproductive to public health.” 0fficials involved with the United States’s Operation Warp Speed—the program tasked with expediting the creation of a vaccine for COVID-19—are discussing the merits of rationing the Moderna vaccine for adults aged 18–55, as is being discussed in the United Kingdom. This would be accomplished either by giving half doses of the first shot or delaying the immunization’s second shot, Moncef Slaoui, the head of OWS, tells CBS News’s “Face the Nation.” Ultimately, the Food and Drug Administration will determine if the vaccines can be given on a different schedule, and a decision has not yet been made. Originally, OWS anticipated that 20 million vaccine doses would have been distributed by the end of 2020. As of January 2, the Centers for Disease Control and Prevention reports that 13 million doses have gone out, and 4.23 million people in the US have received at least one dose, primarily frontline healthcare workers and nursing home residents so far. Going off-schedule for lower-risk adults would allow twice as many people to receive their first shot, Slaoui says. The vaccine’s urgency has become more pronounced as a more contagious strain of SARS-CoV-2 that originated in the UK has now been found in the US. If reducing the volume of the first dose is approved by the FDA, the rationing would only affect the vaccine made by Moderna, Slaoui explains, which he claims would still provide the same protection as a full dose. Officials from Moderna have not yet verified if that is the case. Germany is also deliberating holding off on the second shot to spread around more initial doses, while Denmark has already approved the measure, Reuters reports. Officials from Pfizer and BioNTech released a joint statement last week in response to the UK’s desire to hold off on the second dose from the initial plan of 21 days to as long as 12 weeks after the first. “The safety and efficacy of the vaccine has not been evaluated on different dosing schedules as the majority of trial participants received the second dose within the window specified in the study design,” the statement reads, according to Reuters. Anthony Fauci, the head of the National Institute of Allergy and Infectious Diseases, along with other medical experts around the globe, say they disagree that rationing would be effective, The New York Times reports. Fauci says that the US will not be following the UK’s lead to delay the second dose, should the British government decide to do that. Still, it will be down to the FDA to decide if altered dosing will be permitted. Speaking on CNN, Leana Wen, the former health commissioner of Baltimore, agrees with Fauci’s take. “I understand the desire to stretch out the doses that we have and to say, ‘Hey, if one dose can offer some partial protection, why not give it to as many people as we can?’ The problem, though, is that is not how the studies were conducted.”

|
Scooped by
Juan Lama
|
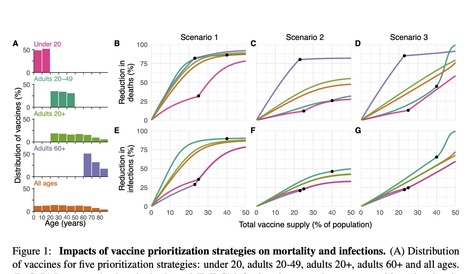
Limited initial supply of SARS-CoV-2 vaccine raises the question of how to prioritize available doses. Here, we used a mathematical model to compare five age-stratified prioritization strategies. A highly effective transmission-blocking vaccine prioritized to adults ages 20-49 years minimized cumulative incidence, but mortality and years of life lost were minimized in most scenarios when the vaccine was prioritized to adults over 60 years old. Use of individual-level serological tests to redirect doses to seronegative individuals improved the marginal impact of each dose while partially addressing existing inequities in COVID-19 impact. While maximum impact prioritization strategies were broadly consistent across countries, transmission rates, vaccination rollout speeds, and estimates of naturally acquired immunity, this framework can be used to compare impacts of prioritization strategies across contexts. Available in medRxiv (Dec. 7, 2020): https://doi.org/10.1101/2020.09.08.20190629
|



 Your new post is loading...
Your new post is loading...