Your new post is loading...

|
Scooped by
Juan Lama
|
The Omicron variant is characterised by more than 50 distinct mutations, the majority of which are located in the spike protein. The implications of these mutations for disease transmission, tissue tropism and diagnostic testing are still to be determined. We evaluated the relative performance of saliva and mid-turbinate swabs as RT-PCR samples for the Delta and Omicron variants. The positive percent agreement (PPA) of saliva swabs and mid-turbinate swabs to a composite standard was 71% (95% CI: 53-84%) and 100% (95% CI: 89-100%), respectively, for the Delta variant. However, for the Omicron variant saliva and mid-turbinate swabs had a 100% (95% CI: 90-100%) and 86% (95% CI: 71-94%) PPA, respectively. This finding supports ex-vivo data of altered tissue tropism from other labs for the Omicron variant. Reassessment of the diagnostic testing standard-of-care may be required as the Omicron variant becomes the dominant variant worldwide. Preprint available at medRxiv (Dec. 24, 2021): https://doi.org/10.1101/2021.12.22.21268246

|
Scooped by
Juan Lama
|
Compared with nasal swabs, saliva tests may better reflect infection deep in the lungs. To the known risk factors for developing severe COVID-19—age, male sex, or any of a series of underlying conditions—a new study adds one more: high levels of the virus in your saliva. Standard COVID-19 tests sample the nasal passage. But several new tests look for SARS-CoV-2, the pandemic coronavirus, in saliva, and the new work finds a striking correlation between high virus levels there and later hospitalization or death. If the results are confirmed, saliva tests could help doctors prioritize which patients in the early stages of the disease should receive medicines that drive down levels of the virus. “I thought it was pretty striking,” says Shane Crotty, a virologist at the La Jolla Institute for Immunology, who was not involved with the research. Crotty notes the results suggest virus levels in saliva reflect viral load deep in the lungs, where the disease does much of its damage in severe cases. “That is a fundamentally valuable insight,” Crotty says. The new work isn’t the first to link the body’s coronavirus load and disease outcome. Several research groups have found a correlation between high viral levels in the nasal passages at the time of a patient’s hospital admission and ultimate disease severity. But other groups have failed to find that same link. The standard test to detect SARS-CoV-2 samples nasal mucus using nasopharyngeal (NP) swabs. The procedure is unpleasant, but it is the customary way to sample respiratory pathogens. In recent months, however, several research groups have developed and received emergency use authorization from the U.S. Food and Drug Administration for tests detecting SARS-CoV-2 in saliva. Yale University researchers were among the first, and the university’s hospitals have been using both saliva and NP swab tests. In both cases, labs analyze the samples using quantitative reverse transcription polymerase chain reaction tests, which can detect genetic material from SARS-CoV-2 and quantify the number of viral particles in each milliliter of sample. Researchers led by Akiko Iwasaki, an immunologist at Yale, compared viral loads in saliva and NP swabs from 154 patients and 109 people without the virus. They divided the patients into groups that had low, medium, and high viral loads as determined by both types of test. Then they compared those results with the severity of symptoms the patients developed later. They found that patients who developed severe disease, were hospitalized, or died were more likely to have had high virus loads in their saliva tests, but not in their NP swabs. Viral load in both saliva and nasal mucus declined over time in patients who recovered, but not in those who died. When Iwasaki and her colleagues reviewed patients’ electronic medical records for markers of disease in the blood, they found that high saliva viral loads correlated with high levels of immune signals such as cytokines and chemokines, nonspecific molecules that ramp up in response to viral infections and have been linked to tissue damage. People with more virus in their saliva also gradually lost certain cells that mount an immune response against viral targets, had lower levels of antibodies targeting the spike protein that the virus uses to enter cells, and were slower to develop the strong immune response needed to knock down the virus in cases where they recovered. The team’s results appeared on 10 January in a preprint that has not been peer reviewed. Iwasaki and her colleagues argue that saliva may be a better predictor of disease outcome than nasal mucus because the latter comes from the upper respiratory tract, whereas severe disease is associated with damage deep in the lungs. “Saliva may better represent what is going on in the lower respiratory tract,” Iwasaki says, because cilia lining the respiratory tract naturally move mucus up from the lungs into the throat, where it mixes with saliva; coughs have the same effect. The results don’t have enough statistical power to reveal how much more likely a person with a high saliva viral load is to develop severe COVID-19, Iwasaki says. She is also eager for other groups to replicate the results, especially because efforts to link high NP swab viral loads with disease progression have had mixed results. If other research confirms the finding, “it would clear away a lot of the fog” around this disease, Crotty says. Monica Gandhi, an infectious disease expert at the University of California, San Francisco, adds that if saliva tests are predictive, they could help doctors identify patients to treat early with either antibodies to reduce viral load or steroids to tamp down overactive nonspecific immune responses. Saliva tests are cheaper and easier than NP tests, but much less widely available. So confirmation of the new results could bolster efforts to make saliva tests more readily available, says Sri Kosuri, CEO of Octant, Inc., a biotech company. “If this study happened in March, we’d be talking about whether we should be doing NP testing at all,” Kosuri says. Preprint available in medRxiv (Jan. 10, 2021): https://doi.org/10.1101/2021.01.04.21249236

|
Scooped by
Juan Lama
|
The YSPH-developed test, called SalivaDirect, has been used as part of an NBA testing program and will now be available to more diagnostic laboratories. A saliva-based laboratory diagnostic test developed by researchers at the Yale School of Public Health to determine whether someone is infected with the novel coronavirus has been granted an emergency use authorization by the U.S. Food and Drug Administration (FDA). The method, called SalivaDirect, is being further validated as a test for asymptomatic individuals through a program that tests players and staff from the National Basketball Association (NBA). SalivaDirect is simpler, less expensive, and less invasive than the traditional method for such testing, known as nasopharyngeal (NP) swabbing. Results so far have found that SalivaDirect is highly sensitive and yields similar outcomes as NP swabbing. With the FDA’s emergency use authorization, the testing method is immediately available to other diagnostic laboratories that want to start using the new test, which can be scaled up quickly for use across the nation — and, perhaps, beyond — in the coming weeks, the researchers said. A key component of SalivaDirect, they note, is that the method has been validated with reagents and instruments from multiple vendors. This flexibility enables continued testing if some vendors encounter supply chain issues, as experienced early in the pandemic. “This is a huge step forward to make testing more accessible,” said Chantal Vogels, a Yale postdoctoral fellow, who led the laboratory development and validation along with Doug Brackney, an adjunct assistant clinical professor. “This started off as an idea in our lab soon after we found saliva to be a promising sample type of the detection of SARS-CoV-2, and now it has the potential to be used on a large scale to help protect public health. We are delighted to make this contribution to the fight against coronavirus.” The preprint on the development and validation of SalivaDirect was recently posted on medRxiv. Development of SalivaDirect as a means of rapidly expanding SARS-CoV-2 testing was spearheaded this spring by Nathan Grubaugh and Anne Wyllie, assistant professor and associate research scientist, respectively, at Yale School of Public Health. After finding saliva to be a promising sample type for SARS-CoV-2 detection, they wanted to improve the method further. “With saliva being quick and easy to collect, we realized it could be a game-changer in COVID-19 diagnostics,” said Wyllie. With testing urgently needed, the Yale team was determined to decrease both testing times and costs, to make testing widely accessible. "Wide-spread testing is critical for our control efforts. We simplified the test so that it only costs a couple of dollars for reagents, and we expect that labs will only charge about $10 per sample. If cheap alternatives like SalivaDirect can be implemented across the country, we may finally get a handle on this pandemic, even before a vaccine,” said Grubaugh. One of the team’s goals was to eliminate the expensive saliva collection tubes that other companies use to preserve the virus for detection. In a separate study led by Wyllie and the team at the Yale School of Public Health, and recently published on medRxiv, they found that SARS-CoV-2 is stable in saliva for prolonged periods at warm temperatures, and that preservatives or specialized tubes are not necessary for collection of saliva.... Validation study available at medRxiv: https://doi.org/10.1101/2020.08.03.20167791 Test's website and details at: https://covidtrackerct.com/about-salivadirect/

|
Scooped by
Juan Lama
|
The FDA authorized its first COVID-19 diagnostic test that allows a person to collect a simple saliva sample themselves, without leaving their home, similar to a personal DNA test. The green light expands upon a previous authorization for a test developed by Rutgers University, designed to use a person’s spit instead of a nasal swab. The agency also granted its blessing to LabCorp’s at-home swab test in late April. “Authorizing additional diagnostic tests with the option of at-home sample collection will continue to increase patient access to testing for COVID-19,” FDA Commissioner Stephen Hahn said in a statement. “This provides an additional option for the easy, safe and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site.” “The FDA has authorized more than 80 COVID-19 tests and adding more options for at-home sample collection is an important advancement in diagnostic testing during this public health emergency,” Hahn added. Rutgers’ prescription-only molecular test currently remains as the only diagnostic authorized by the FDA to process saliva samples. After using a collection kit made by Spectrum DNA, the sealed package is then mailed to the New Jersey university’s clinical genomics lab for analysis. Previously, the university said that quickly administered, saliva-based tests could find equally important use in the field—not just in the home—by sparing healthcare workers exposure from collection methods that require them to manually swab a person’s deep nasal cavity....

|
Scooped by
Juan Lama
|
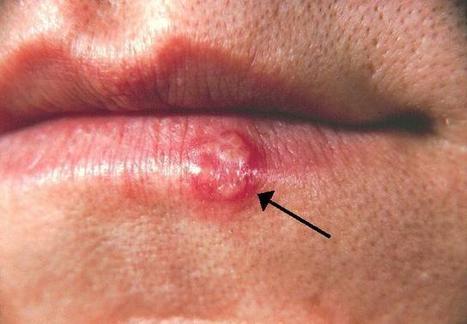
Oral herpes is a highly prevalent infection caused by herpes simplex virus 1 (HSV-1). After an initial infection of the oral cavity, HSV-1 remains latent in sensory neurons of the trigeminal ganglia. Episodic reactivation of the virus leads to the formation of mucocutaneous lesions (cold sores), but asymptomatic reactivation accompanied by viral shedding is more frequent and allows virus spread to new hosts. HSV-1 DNA has been detected in many oral tissues. In particular, HSV-1 can be found in periodontal lesions and several studies associated its presence with more severe periodontitis pathologies. Since gingival fibroblasts may become exposed to salivary components in periodontitis lesions, we analyzed the effect of saliva on HSV-1 and -2 infection of these cells. We observed that human gingival fibroblasts can be infected by HSV-1. However, pre-treatment of these cells with saliva extracts from some but not all individuals led to an increased susceptibility to infection. Furthermore, the active saliva could expand HSV-1 tropism to cells that are normally resistant to infection due to the absence of HSV entry receptors. The active factor in saliva was partially purified and comprised high molecular weight complexes of glycoproteins that included secretory Immunoglobulin A. Interestingly, we observed a broad variation in the activity of saliva between donors suggesting that this activity is selectively present in the population. The active saliva factor, has not been isolated, but may lead to the identification of a relevant biomarker for susceptibility to oral herpes. The presence of a salivary factor that enhances HSV-1 infection may influence the risk of oral herpes and/or the severity of associated oral pathologies. Published on PLOS One on October 3, 2019: https://doi.org/10.1371/journal.pone.0223299
|

|
Scooped by
Juan Lama
|
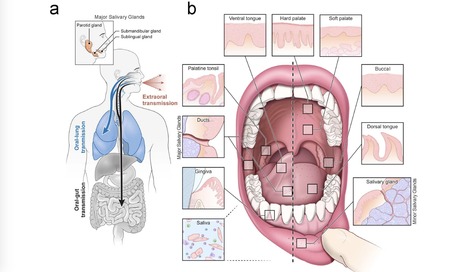
Despite signs of infection—including taste loss, dry mouth and mucosal lesions such as ulcerations, enanthema and macules—the involvement of the oral cavity in coronavirus disease 2019 (COVID-19) is poorly understood. To address this, we generated and analyzed two single-cell RNA sequencing datasets of the human minor salivary glands and gingiva (9 samples, 13,824 cells), identifying 50 cell clusters. Using integrated cell normalization and annotation, we classified 34 unique cell subpopulations between glands and gingiva. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral entry factors such as ACE2 and TMPRSS members were broadly enriched in epithelial cells of the glands and oral mucosae. Using orthogonal RNA and protein expression assessments, we confirmed SARS-CoV-2 infection in the glands and mucosae. Saliva from SARS-CoV-2-infected individuals harbored epithelial cells exhibiting ACE2 and TMPRSS expression and sustained SARS-CoV-2 infection. Acellular and cellular salivary fractions from asymptomatic individuals were found to transmit SARS-CoV-2 ex vivo. Matched nasopharyngeal and saliva samples displayed distinct viral shedding dynamics, and salivary viral burden correlated with COVID-19 symptoms, including taste loss. Upon recovery, this asymptomatic cohort exhibited sustained salivary IgG antibodies against SARS-CoV-2. Collectively, these data show that the oral cavity is an important site for SARS-CoV-2 infection and implicate saliva as a potential route of SARS-CoV-2 transmission. Published in Nature Medicine (March 25, 2021): https://doi.org/10.1038/s41591-021-01296-8

|
Scooped by
Juan Lama
|
Saliva Specimens to Detect SARS-CoV-2 Infection In this letter, the investigators report that saliva specimens and nasopharyngeal swab specimens had similar sensitivity in the detection of COVID-19. Rapid and accurate diagnostic tests are essential for controlling the ongoing Covid-19 pandemic. Although the current standard involves testing of nasopharyngeal swab specimens by quantitative reverse-transcriptase polymerase chain reaction (RT-qPCR) to detect SARS-CoV-2, saliva specimens may be an alternative diagnostic sample. Rigorous evaluation is needed to determine how saliva specimens compare with nasopharyngeal swab specimens with respect to sensitivity in detection of SARS-CoV-2 during the course of infection. A total of 70 inpatients with Covid-19 provided written informed consent to participate in our study available with the full text of this letter at NEJM.org). After Covid-19 was confirmed with a positive nasopharyngeal swab specimen at hospital admission, we obtained additional samples from the patients during hospitalization. We tested saliva specimens collected by the patients themselves and nasopharyngeal swabs collected from the patients at the same time point by health care workers. Using primer sequences from the Centers for Disease Control and Prevention, we detected more SARS-CoV-2 RNA copies in the saliva specimens (mean log copies per milliliter, 5.58; 95% confidence interval [CI], 5.09 to 6.07) than in the nasopharyngeal swab specimens (mean log copies per milliliter, 4.93; 95% CI, 4.53 to 5.33). In addition, a higher percentage of saliva samples than nasopharyngeal swab samples were positive up to 10 days after the Covid-19 diagnosis. At 1 to 5 days after diagnosis, 81% (95% CI, 71 to 96) of the saliva samples were positive, as compared with 71% (95% CI, 67 to 94) of the nasopharyngeal swab specimens. These findings suggest that saliva specimens and nasopharyngeal swab specimens have at least similar sensitivity in the detection of SARS-CoV-2 during the course of hospitalization... Published in New England J. Medicine (August 28, 2020): https://doi.org/10.1056/NEJMc2016359

|
Scooped by
Juan Lama
|
A quick, cheap and painless test that detects SARS-CoV-2 RNA in spit could be used for mass testing. Chantal Vogels at Yale School of Medicine in New Haven, Connecticut, and colleagues developed a simple saliva test — called SalivaDirect — to address the growing demand for extensive testing as lockdowns lift (C. B. F. Vogels et al. Preprint at medRxiv http://doi.org/d5s3; 2020). Compared with the gold-standard nose and throat swab, the saliva test is less invasive, does not need to be conducted by a trained professional and avoids the use of scarce chemicals that are needed to store and extract viral RNA. In validation experiments, SalivaDirect detected 32 out of 34 samples that tested positive in nose and throat swabs, and 30 out of 33 negative samples. The researchers estimate a cost-per-spit of US$1.29–$4.37, and have requested that the United States Food and Drug Administration authorize the test for emergency use. Preprint of study available at medRxiv (August 4, 2020): https://doi.org/10.1101/2020.08.03.20167791

|
Scooped by
Juan Lama
|
The search for SARS-CoV-2 RNA in 60 saliva samples yielded the same results as conventional nasal swab tests taken from the same patients. There are now more options for COVID-19 testing as the US Food and Drug Administration gave emergency use authorization on April 13 for a saliva-based test, providing an alternative to the swab testing currently performed. “You want to be in all types of situations with all types of options so that we can have as much testing as possible in whatever form is suitable,” Amesh Adalja, who works on infectious disease and pandemic preparedness at Johns Hopkins University and is not involved with the development of the test, tells the Associated Press. Currently, testing for COVID-19 involves a healthcare professional inserting a swab into each nostril, one at a time, to the nasopharynx at the back of the nasal cavity, gently scraping the tissue to collect material, and sending off for analysis, according to UC Davis Health. This method is cumbersome as it needs to be done by a qualified worker wearing fresh gloves and other personal protective equipment (PPE), which are in short supply. Additionally, many areas are experiencing a lack of tests available or a large backlog of samples to process. The collection of a saliva sample requires spitting into a tube, resulting in a much less invasive procedure without tying up large amounts of PPE. Per the Food and Drug Administration’s (FDA) instructions, the testing would still occur in a healthcare setting under the supervision of a qualified professional.... Performance Letter by FDA: https://www.fda.gov/media/136875/download
|



 Your new post is loading...
Your new post is loading...