Your new post is loading...

|
Scooped by
Juan Lama
|
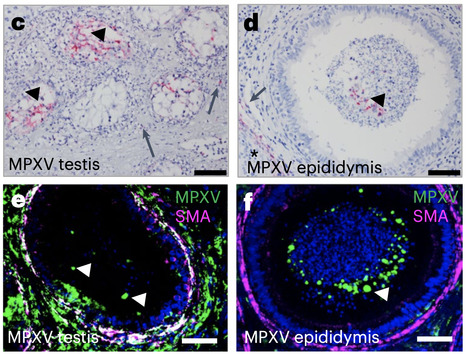
Close contact through sexual activity has been associated with the spread of monkeypox virus (MPXV) in the ongoing, global 2022 epidemic. However, it remains unclear whether MPXV replicates in the testes or is transmitted via semen to produce an active infection. We carried out a retrospective analysis of MPXV-infected crab-eating macaque archival tissue samples from acute and convalescent phases of infection of clade I or clade II MPXV using immunostaining and RNA in situ hybridization. We detected MPXV in interstitial cells and seminiferous tubules of testes as well as epididymal lumina, which are the sites of sperm production and maturation. We also detected inflammation and necrosis during the acute phase of the disease by histological analysis. Finally, we found that MPXV was cleared from most organs during convalescence, including healed skin lesions, but could be detected for up to 37 d post-exposure in the testes of convalescent macaques. Our findings highlight the potential for sexual transmission of MPXV in humans. Detection of monkeypox viruses (MPXV) in archival testes samples from acute and convalescent MPXV-infected macaques provides evidence supporting the potential for sexual transmision of MPXV. Published (Oct. 17, 2022) in Nature Microbiology: https://doi.org/10.1038/s41564-022-01259-w

|
Scooped by
Juan Lama
|
Genome analyses suggesting virus persistence raise worries about stigmatization of survivors. An Ebola outbreak in Guinea that has so far sickened at least 18 people and killed nine has stirred difficult memories of the devastating epidemic that struck the West African country between 2013 and 2016, along with neighboring Liberia and Sierra Leone, leaving more than 11,000 people dead. But it may not just be the trauma that has persisted. The virus causing the new outbreak barely differs from the strain seen 5 to 6 years ago, genomic analyses by three independent research groups have shown, suggesting the virus lay dormant in a survivor of the epidemic all that time. “This is pretty shocking,” says virologist Angela Rasmussen of Georgetown University. “Ebolaviruses aren’t herpesviruses”—which are known to cause long-lasting infections—“and generally RNA viruses don’t just hang around not replicating at all.” Scientists knew the Ebola virus can persist for a long time in the human body; a resurgence in Guinea in 2016 originated from a survivor who shed the virus in his semen more than 500 days after his infection and infected a partner through sexual intercourse. “But to have a new outbreak start from latent infection 5 years after the end of an epidemic is scary and new,” says Eric Delaporte, an infectious disease physician at the University of Montpellier who has studied Ebola survivors and is a member of one of the three teams. Outbreaks ignited by Ebola survivors are still very rare, Delaporte says, but the finding raises tricky questions about how to prevent them without further stigmatizing Ebola survivors. The current outbreak in Guinea was detected after a 51-year-old nurse who had originally been diagnosed with typhoid and malaria died in late January. Several people who attended her funeral fell ill, including members of her family and a traditional healer who had treated her, and four of them died. Researchers suspected Ebola might have caused all of the deaths, and in early February they discovered the virus in the blood of the nurse’s husband. An Ebola outbreak was officially declared on 13 February, with the nurse the likely index case. The Guinea Center for Research and Training in Infectious Diseases (CERFIG) and the country’s National Hemorrhagic Fever Laboratory have each read viral genomes from four patients; researchers at the Pasteur Institute in Dakar, Senegal, sequenced two genomes. In three postings today on the website virological.org, the groups agree the outbreak was caused by the Makona strain of a species called Zaire ebolavirus, just like the past epidemic. A phylogenetic tree shows the new virus falls between virus samples from the 2013–16 epidemic. Until recently, scientists assumed Ebola epidemics start when a virus jumps species, from an animal host to humans. Theoretically, that could have happened in Guinea, says virologist Stephan Günther of the Bernhard Nocht Institute for Tropical Medicine, who worked with one of the three teams. But given the similarity between viruses from the epidemic and the new ones, “It must be incredibly unlikely.” Outside scientists agree but say it hasn’t been proved that Ebola lay dormant in one person for 5 years. “From the tree, you’d conclude that it is a virus that persisted in some way in the area, and sure, most likely in a survivor,” says Dan Bausch, a veteran of several Ebola outbreaks who leads the United Kingdom’s Public Health Rapid Support Team. But it is hard to rule out scenarios such as a small, unrecognized chain of human to human transmission, Bausch adds: “For example, a 2014 survivor infects his wife a few years after recovery, who infects another male, who survives and carries virus for a few years, then infecting another women, who is then seen by a nurse who dies”—the index case in the new outbreak. The nurse was not known to be a survivor herself, but she could have had contact with a survivor privately or through her job, or she might have been infected herself years ago with few symptoms. “Figuring out what exactly happened is one of the biggest questions now,” Bausch says. Another ongoing outbreak of Ebola in North Kivu, in the Democratic Republic of the Congo, was also started by transmission from someone infected during a previous outbreak, Delaporte notes. (The survivor had tested negative for Ebola twice after his illness in 2020.) Taken together, that suggests humans are now as likely to be the source of a new outbreak of Ebola as wildlife, he says. “This is clearly a new paradigm for how these outbreaks start.” Outbreaks sparked by survivors may even become more likely, now that increasing mobility and other factors have caused each eruption of Ebola to become bigger, resulting in more survivors, says Fabian Leendertz, a wildlife veterinarian who was involved in the sequencing. The cases raise important new research questions, Bausch says: “How do we need to change our response to escape from the cycle of outbreak-response-reintroduction-outbreak?” he asks. “Can we use new therapeutics to clear virus from survivors?” But the most immediate question is what these results mean for Ebola survivors, who face a lot of hardship already. Many have not only lost friends and family to the virus, but also struggle with long-term aftereffects, such as muscle pains and eye problems. In a study published in February, Delaporte found that about half of more than 800 Ebola survivors in Guinea still reported symptoms 2 years after their illness, and one-quarter after 4 years. On top of this, survivors have faced intense stigmatization. Many conspiracy theories swirled in the aftermath of the epidemic, including the claim that survivors had sold family members to international organizations to save themselves, says Frederic Le Marcis, a social anthropologist at the École Normale Supérieure of Lyon and the French Research Institute for Development, who is working in Guinea. One man, he says, was the only one to survive out of 11 family members and when he came back, no one wanted to work with him. “He was seen as someone untrustworthy.” News that a survivor likely touched off the current outbreak could cause further problems for survivors, Le Marcis says: “Will they be highlighted as a source of danger? Will they be chased out of their own families and communities?” Alpha Keita, a virologist who led the sequencing work at CERFIG, worries about stigmatization and even violence against survivors have occupied him since he first got the surprising results a week ago. One important message to the public should be that some people infected with Ebola show few symptoms, meaning people may be survivors without knowing it. “So don’t stigmatize Ebola survivors—you don’t know that you are not a survivor yourself,” Keita says. Bausch calls for an educational campaign explaining that unprotected sex with an Ebola survivor may pose a risk, but casual contacts such as shaking hands and working together do not. And although there needs to be some medical monitoring of survivors, it cannot just be about testing them for Ebola virus, he says. “We need to recognize and assist with all the other challenges, physical, mental, and social, that survivors and their families face.” The key, Bausch says, is to “not just treat survivors as some hot potato risk of starting another outbreak.” It also presents a challenge to the country’s health care system if every patient with fever and diarrhea has to be a considered potential Ebola case, Le Marcis says. Fortunately, Ebola vaccines and treatments have become available in recent years. Already, several thousand contacts of the new Ebola patients, and contacts of these contacts, have been vaccinated. Health care workers are being immunized as well. Vaccinating survivors might even help clear latent infections, Rasmussen says. And the fact that viral samples were sequenced in Guinea this time around shows the country’s scientific capabilities have improved, Delaporte says: “Seven years ago, when the epidemic started, there was no infrastructure in Guinea to be able to do this.” Sequences of the EBOV strain available in Virological (March 12, 2021): https://virological.org/t/guinea-2021-ebov-genomes/651

|
Scooped by
Juan Lama
|
Spanish health authorities have confirmed a case of a man spreading dengue through sex, a world first for a virus which until recently was thought to be transmitted only by mosquitos. The case concerns a 41-year-old man from Madrid who contracted dengue after having sex with his male partner who picked up the virus from a mosquito bite during a trip to Cuba, said Susana Jimenez of the Madrid region's public health department. His dengue infection was confirmed in September and it puzzled doctors because he had not travelled to a country where the disease, which causes severe flu-like symptoms such as high fever and body aches, is common, she added. "His partner presented the same symptoms as him but lighter around ten days earlier, and he had previously visited Cuba and the Dominican Republic," she said. "An analysis of their sperm was carried out and it revealed that not only did they have dengue but that it was exactly the same virus which circulates in Cuba." A "likely' case of sexual transmission of dengue between a man and a woman was the subject of a recent scientific article in South Korea, she said. In an e-mail sent to AFP, the Stockholm-based European Centre for Disease Prevention and Control (ECDC), which monitors health and disease in Europe, said this was "to our knowledge, the first sexual transmission of the dengue virus among men who have sex with men." Dengue is transmitted mainly by the Aedes Aegypti mosquito, which thrives in densely-populated tropical climates and breeds in stagnant pools of water. It kills 10,000 people a year and infects over 100 million. The disease is fatal only in extreme cases but the symptoms are extremely unpleasant, including a high fever, severe headaches and vomiting. It also exacts a heavy economic burden on countries as sufferers are unable to work, as well as overwhelming health services when there is a severe outbreak. It is most serious - and deadly - in children, especially young girls. Scientists do not know why. Dengue is most commonly caught by people traveling to hotter climates such as South East Asia, Africa, Australia, the Caribbean and South and Central America.
|

|
Scooped by
Juan Lama
|
The patient was a 39-year-old man, who travelled in Austria during the first 2 weeks of May, 2022. He self-identified as an MSM and sex worker and reported condomless sexual intercourse with several male partners during the previous month. The patient was HIV-infected, treated with dolutegravir and lamivudine, with viral suppression and immune recovery, and reported a history of sexually transmitted infections. He was admitted to the hospital 5 days after symptom onset. His symptoms included fever, followed by the appearance of clustered itchy papular lesions in the anal region and single lesions on the head, thorax, legs, arms, hand, and penis. The patient reported one dose of smallpox vaccination during childhood, more than 30 years earlier. He did not receive any current treatment for monkeypox virus infection. Monkeypox virus infection was confirmed by real-time PCR on a skin lesion swab (quantification cycle [Cq] 18·9) and scab (Cq 21·4) collected on day 5 after symptom onset. The virus was successfully isolated in vitro from a swab of a skin lesion on the head (appendix p 2). Plasma, urine, and semen samples were longitudinally collected to monitor the duration of viral shedding (table). Monkeypox virus DNA was detected in plasma collected on day 8 after symptom onset only. Urine samples were negative. Monkeypox virus DNA was detected in all semen samples tested during the period of observation (Cq range 27·8–40·6). Semen collected on day 6 after symptom onset was inoculated in Vero E6 cells (ATCC; Manassas VA, USA). Clear cytopathic effect was observed 48 h after the inoculum and monkeypox virus replication was confirmed by real-time PCR on DNA purified from cell growth medium collected after 48 h, 72 h, and 96 h. .. Published in The Lancet (August 2, 2022):

|
Scooped by
Juan Lama
|
The virus responsible for COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been detected in stool, gastrointestinal tract, saliva, and urine samples.2 However, little is known about SARS-CoV-2 in semen.... A total of 38 patients were enrolled for semen testing. Of these 38 participants who provided a semen specimen, 23 participants (60.5%) had achieved clinical recovery and 15 participants (39.5%) were at the acute stage of infection. Results of semen testing found that 6 patients (15.8%) had results positive for SARS-CoV-2, including 4 of 15 patients (26.7%) who were at the acute stage of infection and 2 of 23 patients (8.7%) who were recovering, which is particularly noteworthy. But there was no significant difference between negative and positive test results for patients by age, urogenital disease history, days since onset, days since hospitalization, or days since clinical recovery.... In this cohort study, we found that SARS-CoV-2 can be present in the semen of patients with COVID-19, and SARS-CoV-2 may still be detected in the semen of recovering patients. Owing to the imperfect blood-testes/deferens/epididymis barriers, SARS-CoV-2 might be seeded to the male reproductive tract, especially in the presence of systemic local inflammation. Even if the virus cannot replicate in the male reproductive system, it may persist, possibly resulting from the privileged immunity of testes. So far, researchers have found 27 viruses associated with viremia in human semen. Published in JAMA (May 7, 2020): See also study in med Rxiv that did not find the virus in semen:

|
Scooped by
Juan Lama
|
Contrary to the long-held view that semen can only act as a way to transmit HIV-1 from men to women, scientists at The Wistar Institute and the University of Puerto Rico found that frequent and sustained semen exposure can change the characteristics of the circulating and vaginal tissue immune cells that are targets for infection, reducing the susceptibility to a future infection. This finding, published in the journal Nature Communications, also provides a potential explanation as to why a small number of female sex workers worldwide continue to test negative for infection despite continuous high-risk sexual activity. "While HIV infection has been with us for more than 30 years, this is the first study that describes how semen exposure over time could result in local tissue changes that limit HIV infection in humans," said Montaner, who is the lead author of the new study. "Apart from defining a new factor that may regulate HIV transmission, this unexpected finding could directly impact the design of future HIV vaccine studies that commonly recruit female sex workers. Currently, condomless sex is assumed to only promote the likelihood of infection. Our observation, however, raises the hypothesis that frequent semen exposure may potentially reduce HIV transmission." In the study, animals were exposed to semen twice a week over 20 weeks with or without inactivated particles of the simian immunodeficiency virus (SIV is an HIV-like virus that infects primates and causes a disease similar to AIDS); after this conditioning period, they received low-dose intravaginal SIV challenges. Semen-exposed animals showed a 42% decrease in the risk of infection. Scientists analyzed specific markers of immune activation in the cervicovaginal microenvironment and in the bloodstream. On circulating CD4+ cells, semen conditioning was associated with lower expression of the CCR5 receptor, which acts as a binding site for HIV to enter its host cells, supporting the observation of a lower susceptibility to SIV vaginal challenge. Furthermore, semen-conditioned animals had higher levels of the CCL5 cytokine, a natural HIV-suppressive factor, in the cervicovaginal compartment in response to SIV challenge. "Importantly, we show that semen exposure can promote host resistance but does not protect against infection," said Montaner. "Therefore, our data do not change the fact that prevention methods, such as condom use and PrEP (pre-exposure prophylaxis) remain our best strategies to prevent infection." Findings reported in the journal Nature Communications in August 21, 2019: https://doi.org/10.1038/s41467-019-11814-5
|



 Your new post is loading...
Your new post is loading...