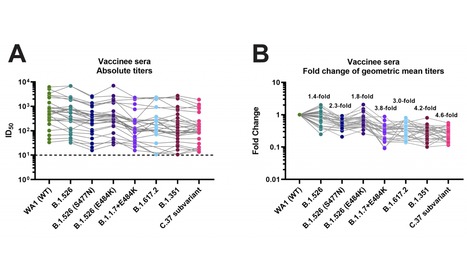
Highly efficacious vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed. However, the emergence of viral variants that are more infectious than the earlier SARS-CoV-2 strains is concerning. Several of these viral variants have the potential to partially escape neutralizing antibody responses warranting continued immune-monitoring. Here, we tested a number of currently circulating viral variants of concern/interest, including B.1.526 (Iota), B.1.1.7+E484K (Alpha), B.1.351 (Beta), B.1.617.2 (Delta) and C.37 (Lambda) in neutralization assays using a panel of post-mRNA vaccination sera. The assays were performed with authentic SARS-CoV-2 clinical isolates in an assay that mimics physiological conditions.
We found only small decreases in neutralization against B.1.526 and an intermediate phenotype for B.617.2. The reduction was stronger against a sub-variant of C.37, followed by B.1.351 and B.1.1.7+E484K. C.37 is currently circulating in parts of Latin America and was detected in Germany, the US and Israel. Of note, reduction in a binding assay that also included P.1, B.1.617.1 (Kappa) and A.23.1 was negligible. Taken together, these findings suggest that mRNA SARS-CoV-2 vaccines may remain effective against these viral variants of concern/interest and that spike binding antibody tests likely retain specificity in the face of evolving SARS-CoV-2 diversity.
Preprint Available in medRxiV (July 23, 2021):



 Your new post is loading...
Your new post is loading...