Your new post is loading...

|
Scooped by
Juan Lama
|
GAITHERSBURG, Md., June 11, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company developing next-generation vaccines for serious infectious diseases, today announced preclinical and clinical data on the company's original recombinant protein COVID-19 vaccine candidate, NVX-CoV2373, and for a new vaccine directed against the SARS-CoV-2 Beta (B.1.351) variant, which was originally identified in South Africa. The data show that the vaccines demonstrated strong immunogenicity and protection against both the Alpha (B.1.1.7) variant, which was originally identified in the United Kingdom, and the Beta (B.1.351) variant as well as the original SARS-CoV-2 in animal and human studies. A preprint of the manuscript, 'Immunogenicity and In vivo protection of a variant nanoparticle vaccine that confers broad protection against emerging SARS-CoV-2 variants,' is available at bioRxiv.org and has been submitted for peer review. "While current vaccines are effective against selected SARS-CoV-2 variant strains, newly emerging variants are also being identified that have the ability to overcome vaccine induced immunity," said Matthew Frieman, PhD, Associate Professor of Microbiology and Immunology at the University of Maryland School of Medicine, who collaborated on these studies. "This work demonstrates that variant vaccines that protect against these newly emerging variants have the potential to be highly effective and may produce broader protection against variants we know of and those that will arise in the future. Clinical trials will provide further evidence on the effectiveness of variant vaccines." The studies compared the Beta (B.1.351)-directed vaccine to Novavax' prototype vaccine candidate as standalone, in combination, and as heterologous prime boost vaccine. The findings show a broad array of cellular and humoral responses in animal models against all virus strains evaluated. The Alpha (B.1.1.7) and Beta (B.1.351) variant strains have created public health concerns due to increased transmission rates and lower efficacy of current vaccines seen against Beta (B.1.351). "These data suggest that not only could one booster dose of this variant-directed vaccine potentially provide a robust, protective immune boost after vaccination against the original SARS-CoV-2 virus, but also the potential to provide broad protection against various virus strains if used as a primary vaccine regimen," said Gregory M. Glenn, M.D., President of Research and Development, Novavax. "This broad immune coverage is vital to controlling the pandemic as variants of concern continue to emerge worldwide that could jeopardize the protection created through ongoing COVID-19 vaccination efforts." Study 1: Immunogenicity and Protection in Mice Mice were immunized with NVX-CoV2373 or rS-B.1.351 alone, in combination, or as a heterologous prime boost. Mice vaccinated with any of the four regimens displayed elevated antibody titers against both the original and Beta (B.1.351) spike. Heterologous or bivalent vaccination is highly immunogenic against the prototype spike compared to monovalent approaches, while immunization with monovalent rS-B.1.351 or bivalent spike resulted in the highest anti-B.1.351 spike IgG titers among regimens tested. Additionally, mice immunized with rS-B.1.351 alone produced elevated neutralizing antibody titers to the Alpha (B.1.1.7) and Beta (B.1.351) strains compared to titers upon immunization with the prototype-directed strain. Titers were similar in the heterologous and bivalent vaccine groups. Antibodies produced after vaccination with rS-B.1.351 inhibited binding between human angiotensin converting enzyme-2 receptor (hACE2) and the variant spike or original spike to the same degree. This indicates that rS-B.1.351 can efficiently protect "backward" against original SARS-CoV-2 strains. In contrast, NVX-CoV2373 was less efficient at protecting "forward" against the Beta (B.1.351) variant strain. Whether immunized with prototype vaccine or rS-B.1.351 alone, in combination, or as a heterologous prime boost, mice were protected when challenged with live Alpha (B.1.1.7) or Beta (B.1.351) variant strains of SARS-CoV-2. Study 2: Anamnestic Response in Baboons A cohort of baboons that were originally immunized with prototype vaccine were boosted approximately one year later with one or two doses of rS-B.1.351. Seven days after the first rS-B.1.351 boost, those that originally received adjuvanted prototype-directed vaccine exhibited a strong immune response, with anti-Spike IgG titers that were higher than the peak immune response observed during the primary immunization series. The rS-B.1.351 boost elicited comparable antibody titers against original and rS-B.1.351 spike. Further, the same cohort exhibited a strong neutralizing response to Alpha (B.1.1.7) and Beta (B.1.351) strains and hACE2-inhibiting antibody response following the boost, despite having undetectable titers before the boost. High neutralizing antibody titers were observed seven days post-boost, demonstrating "a robust, durable antibody response even one year after the primary vaccination series." These results suggest that one dose of a variant-directed vaccine may be sufficient for boosting regimens after previous immunization with a COVID-19 vaccine based on the original spike strain. Study 3: Robust Antibody Response in Humans NVX-CoV2373 is currently being studied in multiple clinical trials, including in locations where Alpha (B.1.1.7) and Beta (B.1.351) variant strains are widespread. Thirty randomly selected human serum samples from Phase 2 clinical trial participants after their second dose of the vaccine were assayed. The sera were analyzed for their ability to neutralize Alpha (B.1.1.7) and Beta (B.1.351) strains. The trial participants' sera demonstrated a neutralizing capacity of the Alpha (B.1.1.7) strain equal to NVX-CoV2373, with a modest reduction in neutralizing capacity against the Beta (B.1.351) strain. These data support the development and production of a Beta (B.1.351) targeted vaccine, which also demonstrated to be efficient at protecting mice against Beta (B.1.351) and to induce a strong response in primates originally immunized with NVX-CoV2373. Furthermore, the data support that a booster vaccine containing a variant strain could both increase antibody levels as well as broaden coverage. Novavax expects to initiate further clinical testing of rS-B.1.351 in the fall of 2021.... See bioRxiv (June 9, 2021): https://doi.org/10.1101/2021.06.08.447631

|
Scooped by
Juan Lama
|
The emergence of SARS-CoV-2 variants with enhanced transmissibility, pathogenesis and resistance to vaccines presents urgent challenges for curbing the COVID-19 pandemic. While Spike mutations that enhance virus infectivity may drive the emergence of these novel variants, studies documenting a critical a role for interferon responses in the early control of SARS-CoV-2 infection, combined with the presence of viral genes that limit these responses, suggest that interferons may also influence SARS-CoV-2 evolution. Here, we compared the potency of 17 different human interferons against 5 viral lineages sampled during the course of the global outbreak that included ancestral and emerging variants. Our data revealed increased interferon resistance in emerging SARS-CoV-2 variants, indicating that evasion of innate immunity is a significant driving force for SARS-CoV-2 evolution. These findings have implications for the increased lethality of emerging variants and highlight the interferon subtypes that may be most successful in the treatment of early infections. Posted in bioRxiv (March 21, 2021): https://doi.org/10.1101/2021.03.20.436257

|
Scooped by
Juan Lama
|
Doctors in France are treating a critically ill patient infected with the South African coronavirus variant, four months after he recovered from COVID-19, in what study authors said was the first case of its kind. The 58-year-old man had a history of asthma and initially tested positive for COVID-19 in September when he presented to medical staff with a fever and shortness of breath. The symptoms persisted only for a few days, and the man tested negative for COVID-19 twice in December 2020. However, he was admitted to hospital in January and diagnosed with the South African variant. The patient's condition worsened, and he is currently in a "critical condition" on a ventilator. "This is, to our knowledge, the first description of reinfection with the South African (variant) causing severe COVID-19, four months after a first mild infection," said authors of a study published this week in the journal Clinical Infectious Diseases. The 501Y.V2 coronavirus variant emerged late last year in South Africa and immediately provoked alarm among disease specialists. It has eight key mutations, one of which affects the virus' spike protein, making it more effective at binding to human cells and therefore more infectious. Vaccine manufacturers Pfizer/BioNTech and Moderna say their mRNA vaccines retain their effectiveness against the South African variants and another that emerged last year in Britain. However a study last week showed that AstraZeneca's vaccine failed to prevent mild and moderate cases of infection of the South African variant. "The impact of 501Y.V2 mutations on the effectiveness of vaccines developed based on earlier SARS-CoV-2 strains is still unknown," said the authors of the reinfection study. Case study published in Clinical Infectious Diseases (Feb. 10, 2021): https://doi.org/10.1093/cid/ciab129

|
Scooped by
Juan Lama
|
An early analysis in Britain found that the vaccine had an efficacy rate of nearly 90 percent. But in a small South Africa trial, the efficacy rate dropped to just under 50 percent. Novavax, a little-known company supported by the U.S. federal government’s Operation Warp Speed, said for the first time on Thursday that its Covid-19 vaccine offered robust protection against the virus. But it also found that the vaccine is not as effective against the fast-spreading variant first discovered in South Africa, another setback in the global race to end a pandemic that has already killed more than 2.1 million people. That could be a problem for the United States, which hours earlier reported its first known cases of the contagious variant in two unrelated people in South Carolina. And it came just days after Moderna and Pfizer said that their vaccines were also less effective against the same variant. Novavax, which makes one of six vaccine candidates supported by Operation Warp Speed last summer, has been running trials in Britain, South Africa, the United States and Mexico. It said Thursday that an early analysis of its 15,000-person trial in Britain revealed that the two-dose vaccine had an efficacy rate of nearly 90 percent there. But in a small trial in South Africa, the efficacy rate dropped to just under 50 percent. Almost all the cases that scientists have analyzed there so far were caused by the variant, known as B.1.351. The data also showed that many trial participants were infected with the variant even after they had already had Covid. “We have the first trial — we are the first to conduct an efficacy trial — in the face of a changing virus,” said Stanley Erck, the president and chief executive of Novavax. He said that researchers expected the variants could change the trial results, but “the amount of change has been a bit of a surprise to everyone.” The South Africa trial was relatively small — with just 4,400 volunteers — and was not designed to come up with a precise estimate of how much protection the vaccine provides. Still, the results were striking enough that the company said it would soon begin testing a new vaccine tailored to protect against the variant from South Africa. “You’re going to have to make new vaccines,” Mr. Erck said. John Moore, a virologist at Weill Cornell Medicine who was not involved in the studies, praised the results. “Fifty percent is not as good as 100, but it’s a damn sight better than zero,” he said, noting that given the strong results in Britain, it was likely very similar in efficacy to the Pfizer and Moderna vaccines. While the Pfizer and Moderna vaccines rely on a newer mRNA technology that has not been used in previous vaccines, Novavax’s candidate employs an older, more established method that relies on injecting coronavirus proteins to provoke an immune response. The fact that three vaccines all appeared to show lowered effectiveness against the variant from South Africa is not encouraging, and the results Novavax announced Thursday were the first to occur outside of a laboratory, testing how well a vaccine worked in people infected with a new variant. Johnson & Johnson is also on the cusp of announcing results of its Covid-19 vaccine trials, and has also tested its candidate in South Africa. The announcement from Novavax raises the stakes for Johnson & Johnson. The company was expected to announce its results as early as last weekend, and the delay has triggered speculation among scientists that the firm has also discovered that its vaccine worked less well in South African trial volunteers who were infected with the variant. In an earnings call on Tuesday, Alex Gorsky, the chief executive officer of the company, said they were looking forward to sharing results from their late-stage trial by early next week. The emergence of several highly contagious variants has complicated efforts to bring the pandemic under control, leading world leaders to shut down travel to places like Britain and South Africa even as the variants already appear to have circled the globe. In the United States, researchers have warned that the variant first identified in Britain, which is believed to be more infectious, could become the dominant form of the virus in this country by March. The United States is well behind other countries in testing for such variants, and the one from South Africa has been found in about 30 countries. But experts have also said there are reasons for optimism, noting that the vaccines remain effective. The best way to combat contagious new variants is to continue vaccination and other public health measures, which will slow the virus’s ability to infect new people and mutate further. “This is really worrisome,” said Dr. Peter Hotez, a vaccine expert at the Baylor College of Medicine and the inventor of a coronavirus vaccine. “We have to have the American people vaccinated by late in the spring or early summer to have any hope in preventing the South African and the U.K. variants from taking over.” Drug makers could update their vaccines and offer new shots at regular intervals, similar to the flu vaccine....... Novavax Press Release (Jan. 28, 2021): https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3
|

|
Scooped by
Juan Lama
|
The NIH on Wednesday announced the start of a phase 1 clinical trial evaluating Moderna’s variant-specific COVID-19 vaccine, saying the shot has been administered to trial participants. Moderna shipped doses of the vaccine — a modified version of its mRNA-1273 vaccine, which has been authorized for use in the U.S. since December — to the NIH last month for testing. The vaccine was tweaked to target the B.1.351 variant, which was first identified in South Africa. According to the NIH, the new trial will enroll 210 healthy adults at four clinical research sites in Atlanta, Cincinnati, Nashville and Seattle. Investigators at the four sites anticipate that the trial will be fully enrolled by the end of April, the NIH said. The modified vaccine will be studied in both vaccinated and unvaccinated adults. The vaccinated adults already received mRNA-1273 last year as part of a clinical trial. They will be randomly assigned to receive either a single booster vaccination of 50 µg of the modified vaccine, mRNA-1273.351, or a single vaccination containing one 25 µg dose of mRNA-1273 and one 25 µg dose of mRNA-1273.351. A separate clinical trial will assess a booster shot of the original vaccine in the remaining vaccinated study participants. According to National Institute of Allergy and Infectious Diseases Director Anthony S. Fauci, MD, the B.1.351 variant has been detected in at least nine U.S. states. The NIAID, which co-developed the vaccine with Moderna, is leading and funding the new trial. “Preliminary data show that the COVID-19 vaccines currently available in the United States should provide an adequate degree of protection against SARS-CoV-2 variants,” Fauci said in the NIH release. “However, out of an abundance of caution, NIAID has continued its partnership with Moderna to evaluate this variant vaccine candidate should there be a need for an updated vaccine.” NIH Press Release (March 31, 2021): https://www.nih.gov/news-events/news-releases/nih-clinical-trial-evaluating-moderna-covid-19-variant-vaccine-begins

|
Scooped by
Juan Lama
|
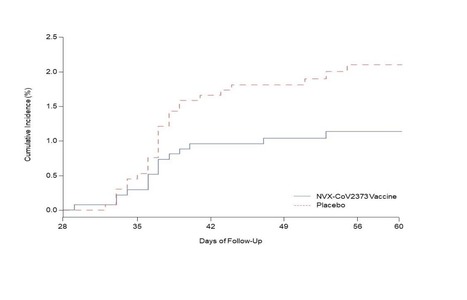
Background The emergence of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) variants threatens progress toward control of the Covid-19 pandemic. Evaluation of Covid-19 vaccine efficacy against SARS-CoV-2 variants is urgently needed to inform vaccine development and use. Methods In this phase 2a/b, multicenter, randomized, observer-blinded, placebo-controlled trial in South Africa, healthy human immunodeficiency virus (HIV)-negative adults (18 to 84 years) or medically stable people living with HIV (PLWH) (18 to 84 years) were randomized in a 1:1 ratio to receive two doses, administered 21 days apart, of either NVX-CoV2373 nanoparticle vaccine (5 micrograms recombinant spike protein with 50 micrograms Matrix-M1 adjuvant) or placebo. The primary endpoints were safety and vaccine efficacy greater than or equal to 7 days following the second dose against laboratory-confirmed symptomatic Covid-19 in previously SARS-CoV-2 uninfected participants. Results A total of 4387 participants were randomized and dosed at least once, 2199 with NVX CoV2373 and 2188 with placebo. Approximately 30% of participants were seropositive at baseline. Among 2684 baseline seronegative participants (94% HIV negative; 6% PLWH), there were 15 and 29 predominantly mild to moderate Covid-19 cases in NVX CoV2373 and placebo recipients, respectively; vaccine efficacy was 49.4% (95% confidence interval [CI]: 6.1 to 72.8). Efficacy in HIV negative participants was 60.1% (95% CI: 19.9 to 80.1), and did not differ by baseline serostatus. Of the primary endpoint cases with available whole genome sequencing, 38 (92.7%) of 41 were the B.1.351 variant. Post-hoc vaccine efficacy against B.1.351 was 51.0% (95% CI: -0.6 to 76.2) in HIV-negative participants. Among placebo recipients, the incidence of symptomatic Covid-19 was similar in baseline seronegative vs baseline seropositive participants during the first 2 months of follow-up (5.3% vs 5.2%). Preliminary local and systemic reactogenicity were primarily mild to moderate and transient, and higher with NVX CoV2373; serious adverse events were rare in both groups. Conclusions The NVX-CoV2373 vaccine was efficacious in preventing Covid-19, which was predominantly mild to moderate and due to the B.1.351 variant, while evidence of prior infection with the presumptive original SARS CoV-2 did not confer protection against probable B.1.351 disease. (Funded by Novavax, The Bill and Melinda Gates Foundation, and the Coalition for Epidemic Preparedness Innovations; ClinicalTrials.gov number, NCT04533399) Preprint available in medRxiv (March 3, 2021): https://doi.org/10.1101/2021.02.25.21252477

|
Scooped by
Juan Lama
|
The bad news, coming nearly a week after a million doses of the AstraZeneca-Oxford vaccine arrived in South Africa, was a big setback for the country. South Africa halted use of the AstraZeneca-Oxford coronavirus vaccine on Sunday after evidence emerged that the vaccine did not protect clinical trial volunteers from mild or moderate illness caused by the more contagious virus variant that was first seen there. The findings were a devastating blow to the country’s efforts to combat the pandemic. Scientists in South Africa said on Sunday that a similar problem held for people who had been infected by earlier versions of the coronavirus: The immunity they acquired naturally did not appear to protect them from mild or moderate cases when they were reinfected by the variant, known as B.1.351. The developments, coming nearly a week after a million doses of the AstraZeneca-Oxford vaccine arrived in South Africa, were an enormous setback for the country, where more than 46,000 people are known to have died from the virus They were also another sign of the dangers posed by new mutations in the coronavirus. The B.1.351 variant has spread to at least 32 countries, including the United States. The number of cases evaluated as part of the studies outlined by South African scientists on Sunday were low, making it difficult to pinpoint just how effective or not the vaccine might be against the variant. And because the clinical trial participants who were evaluated were relatively young and unlikely to become severely ill, it was impossible for the scientists to determine if the variant interfered with the AstraZeneca-Oxford vaccine’s ability to protect against severe Covid-19, hospitalizations or deaths. The scientists said, however, that they believed the vaccine might protect against more severe cases, based on the immune responses detected in blood samples from people who were given it. If further studies show that to be the case, South African health officials will consider resuming use of the AstraZeneca-Oxford vaccine, they said. The new research findings have not been published in a scientific journal. But the discovery that the AstraZeneca-Oxford product showed minimal efficacy in preventing mild and moderate cases of the new variant added to the mounting evidence that B.1.351 makes current vaccines less effective. Pfizer and Moderna have both said that preliminary laboratory studies indicate that their vaccines, while still protective, are less effective against B.1.351. Novavax and Johnson & Johnson have also sequenced test samples from their clinical trial participants in South Africa, where B.1.351 caused the vast majority of cases, and both reported lower efficacy there than in the United States. “These results are very much a reality check,” Shabir Madhi, a virologist at University of the Witwatersrand who ran the AstraZeneca-Oxford vaccine trial in South Africa, said of the findings released on Sunday. The pause in the country’s rollout of the AstraZeneca-Oxford vaccine means that the first shipments will now be put in warehouses. Instead, South African health officials said they would inoculate health workers in the coming weeks with the Johnson & Johnson vaccine, which has shown strong efficacy in preventing severe cases and hospitalizations caused by the new variant. Johnson & Johnson has applied for an emergency use authorization in South Africa. But health officials there indicated that even before it is authorized, some health workers could be given the vaccine as part of an ongoing trial. In the AstraZeneca-Oxford trial in South Africa, roughly 2,000 participants were given either two doses of the vaccine or placebo shots. There was virtually no difference in the numbers of people in the vaccine and placebo groups who were infected with B.1.351, suggesting that the vaccine did little to protect against the new variant. Nineteen of the 748 people in the group that was given the vaccine were infected with the new variant, compared with 20 out of 714 people in the group that was given a placebo. That equates to a vaccine efficacy of 10 percent, though the scientists did not have enough statistical confidence to know for sure whether that figure would hold among more people. Researchers also conducted laboratory experiments on blood samples from people who had been vaccinated and found a significant reduction in the activity levels of vaccine-generated antibodies against the B.1.351 variant compared with other lineages. Beyond the troubling news about the AstraZeneca-Oxford vaccine, Dr. Madhi reported evidence suggesting that past infection by earlier versions of the coronavirus did not protect people in South Africa from the B.1.351 variant. In order to determine who had previously been infected by the coronavirus, researchers tested blood samples from people who had enrolled in a trial of the Novavax vaccine, but who were given placebo shots and not the vaccine itself. The researchers compared the levels of infection by the new variant in people who showed evidence of having previously had Covid-19 with the levels of infection in people who did not, and found no difference. That suggested, Dr. Madhi wrote on a slide presented Sunday night, that “past infection by ‘original’ variants of SARS-CoV-2 do NOT protect against mild and moderate Covid-19 from the B.1.351 variant.....” University of Witwatersrand, Johannesburg (Feb. 7, 2021): https://www.wits.ac.za/covid19/covid19-news/latest/oxford-covid-19-vaccine-trial-results.html
|



 Your new post is loading...
Your new post is loading...