Your new post is loading...

|
Scooped by
Juan Lama
|
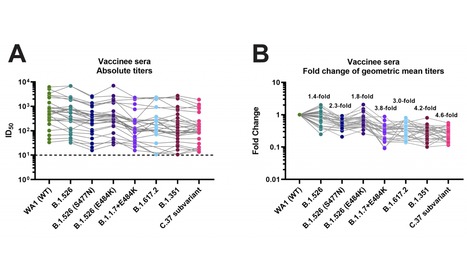
Highly efficacious vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed. However, the emergence of viral variants that are more infectious than the earlier SARS-CoV-2 strains is concerning. Several of these viral variants have the potential to partially escape neutralizing antibody responses warranting continued immune-monitoring. Here, we tested a number of currently circulating viral variants of concern/interest, including B.1.526 (Iota), B.1.1.7+E484K (Alpha), B.1.351 (Beta), B.1.617.2 (Delta) and C.37 (Lambda) in neutralization assays using a panel of post-mRNA vaccination sera. The assays were performed with authentic SARS-CoV-2 clinical isolates in an assay that mimics physiological conditions. We found only small decreases in neutralization against B.1.526 and an intermediate phenotype for B.617.2. The reduction was stronger against a sub-variant of C.37, followed by B.1.351 and B.1.1.7+E484K. C.37 is currently circulating in parts of Latin America and was detected in Germany, the US and Israel. Of note, reduction in a binding assay that also included P.1, B.1.617.1 (Kappa) and A.23.1 was negligible. Taken together, these findings suggest that mRNA SARS-CoV-2 vaccines may remain effective against these viral variants of concern/interest and that spike binding antibody tests likely retain specificity in the face of evolving SARS-CoV-2 diversity. Preprint Available in medRxiV (July 23, 2021): https://doi.org/10.1101/2021.07.21.21260961

|
Scooped by
Juan Lama
|
Data from Qatar provide strongest evidence yet that COVID-19 vaccines can stop strains thought to pose a threat to immunization efforts. Qatar’s second wave of COVID-19 was a double whammy. In January, after months of relatively few cases and deaths, the Gulf nation saw a surge driven by the fast-spreading B.1.1.7 variant, which was first identified in the United Kingdom. Weeks later, the B.1.351 strain, which is linked to reinfections and dampened vaccine effectiveness, took hold. Amid this storm, researchers in Qatar have found some of the strongest evidence yet that current vaccines can quell variants such as B.1.351. Clinical trials in South Africa — where B.1.351 was first identified — had suggested that vaccines would take a hit against such variants. But this study offers a fuller picture of what countries battling such variants can expect. People in Qatar who received two doses of the Pfizer–BioNTech vaccine were 75% less likely to develop a case of COVID-19 caused by B.1.351 than were unvaccinated people, and had near-total protection from severe disease caused by that strain. The findings — published on 5 May in The New England Journal of Medicine1 — suggest that current RNA vaccines are a potent weapon against the most worrisome immune-evading variants. Pfizer, based in New York City, and BioNTech, in Mainz, Germany, are developing an updated RNA vaccine targeting B.1.351, as is Moderna, based in Cambridge, Massachusetts. Early results from Moderna’s efforts suggest that a booster shot of the updated vaccine triggers a strong response against B.1.351. “I think this variant is probably the worst of all the variants we know,” says Laith Jamal Abu-Raddad, an infectious-disease epidemiologist at Weill Cornell Medicine—Qatar in Doha, who led the Qatari study. “We have the tools, despite these variants, to control at least the severe forms of infection — and this should work quite well on transmission.” Weaker protection Researchers in South Africa identified B.1.351 in late 2020, and it’s now the predominant strain there. Laboratory studies show that the variant harbours mutations that blunt the effects of virus-blocking antibodies, and trials suggest that some COVID-19 vaccines are significantly less effective against the strain than against others. Early lab research suggested that RNA vaccines, including the Pfizer–BioNTech jab, would be weakened by B.1.351, but probably not fully compromised. In April, the companies announced that a small trial in South Africa had found the vaccine to be fully effective against B.1.351, but the study of 800 people recorded a total of just 6 infections caused by B.1.351 in the placebo group, so efficacy might have been much lower. Abu-Raddad’s team analysed tens of thousands of COVID-19 cases that occurred between the start of Qatar’s vaccination campaign in late December and the end of March. Genome sequencing showed that B.1.1.7 and B.1.351 were the predominant coronavirus lineages during this period and, from mid-February, each accounted for about half of the country’s cases. The researchers compared SARS-CoV-2 infection rates in vaccinated people with those in unvaccinated controls. People who received two vaccine doses were about 90% less likely to develop an infection caused by B.1.1.7, echoing findings from Israel, the United Kingdom and elsewhere. The researchers identified around 1,500 ‘breakthrough’ infections caused by the B.1.351 variant in vaccinated individuals, but only 179 of these occurred more than 2 weeks after the second dose. There were hardly any severe cases of COVID-19 caused by either B.1.1.7 or B.1.351 among fully vaccinated individuals. “Even though there were breakthrough infections, they didn’t lead to hospitalization and death, except very, very rarely,” says Abu-Raddad. Two people died of COVID-19 caused by B.1.351 after receiving their second vaccine dose, but it is very likely that they were infected before the protective effects of the booster shot began. “If, a year ago, I told somebody we would have 75% effectiveness against the worst variants we had, they would consider this extremely good news,” Abu-Raddad adds. Promising data Shabir Madhi, a vaccinologist at the University of the Witwatersrand in Johannesburg, South Africa, says the Qatari results are promising. The comparatively high levels of virus-blocking antibodies triggered by two doses of an RNA vaccine probably explain why it confers better protection against B.1.351 than do other vaccines, such as the one developed by the University of Oxford, UK, and pharmaceutical company AstraZeneca in Cambridge, UK. But Madhi expects that other vaccines will also prevent severe disease caused by that variant. In another 5 May New England Journal of Medicine study2, his team reported that the jab produced by biotechnology company Novavax in Gaithersburg, Maryland, lowered the risk of getting COVID-19 by 60% in participants without HIV in a South African trial involving more than 6,000 people. As-yet unpublished data show that the vaccine was highly effective against severe cases of COVID-19 caused by B.1.351, with no cases in vaccinated individuals and five in the placebo arm. If vaccine efficacy is lower against B.1.351, even highly successful immunization programmes in countries affected by the variant might not reduce cases to the same extent as in countries dealing with less troublesome strains, says Madhi. “Nevertheless, by protecting high-risk individuals, we could still return to a relatively normal lifestyle, even with ongoing circulation.” Qatar, where more than one-third of the population has received at least one dose of the vaccine, might provide an early glimpse at how the worst coronavirus variants can be controlled. Abu-Raddad says there is evidence that the Pfizer–BioNTech vaccine might also be highly effective at blocking transmission of B.1.351. And after cases of the variant peaked in mid-April, he says, “things have been going extremely well, the numbers are going down very, very rapidly”. Publication cited available in NEJM (May 5, 2021): https://www.nejm.org/doi/10.1056/NEJMc2104974 See also The Lancet (May 5, 2021): https://doi.org/10.1016/S0140-6736(21)00947-8

|
Scooped by
Juan Lama
|
Pfizer Inc and BioNTech said on Thursday their COVID-19 vaccine is around 91% effective at preventing the disease, citing updated trial data that included participants inoculated for up to six months. The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said.The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said. While the new overall efficacy rate of 91.3% is lower than the 95% originally reported in November for its 44,000-person trial, a number of variants have become more prevalent around the world since then. Pfizer’s Chief Executive Officer Albert Bourla said the updated result, which includes data on more than 12,000 people fully inoculated for at least six months, positions the drugmakers to submit for full U.S. regulatory approval. The vaccine is currently authorized on an emergency basis by the U.S. Food and Drug Administration. The trial data “provide the first clinical results that a vaccine can effectively protect against currently circulating variants, a critical factor to reach herd immunity and end this pandemic for the global population,” Ugur Sahin, chief executive officer at BioNTech, said in a statement. Experts fear new variants of COVID-19 from South Africa and Brazil may be resistant to existing vaccines and treatment. More than 300 cases of the South African variant have been detected in more than 25 U.S. states and jurisdictions, according to federal data. Lab tests have previously indicated that BioNTech’s vaccine was less potent but still offered a robust defense against the B1351 variant that first emerged in South Africa. Still, BioNTech reiterated this week there would likely be a future need for booster shots that specifically address new variants and that the group was preparing to upgrade its vaccine when needed. BioNTech has said that it started testing a modified vaccine version against the South African mutant in March for early indications on safety and efficacy but a product for later market launch would require yet another redesign and more tests. The updated trial data would not prompt the company to change that development strategy, a BioNTech spokeswoman said. Pfizer's Press Release (April 1, 2021): https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-confirm-high-efficacy-and-no-serious
|

|
Scooped by
Juan Lama
|
The SARS-CoV-2 B.1.617.2 Variant of Concern (VOC), first detected in India, is now dominant in the UK, having rapidly displaced the B.1.1.7 strain that emerged in the UK with the second COVID-19 wave in late 2020. The efficacy of currently licensed COVID-19 vaccines against B.1.617.2 is unknown; although it possesses 12 mutations in its spike protein relative to the wildtype SARS-CoV-2 first detected in Wuhan, China, in December, 2019, B.1.617.2 lacks mutations at amino acid positions 501 or 484 in its ACE2 receptor-binding domain, commonly associated with VOCs (appendix p 2) or escape from neutralising antibodies (NAbs). To determine vaccine-induced NAb escape by B.1.617.2 and compare activity to previous strains with existing estimates for population-based vaccine efficacy, we carried out an initial analysis of the Legacy study, established in January, 2021, by University College London Hospital and the Francis Crick Institute in London, UK, to track serological responses to vaccination in prospectively recruited staff volunteers (appendix p 6). A detailed description of the methods, including the clinical cohort, virus culture conditions, genetic sequencing, and neutralisation assays, and the statistical analysis are available in the appendix (p 8). The Legacy study was approved by London Camden and Kings Cross Health Research Authority Research and Ethics committee (IRAS number 286469) and sponsored by University College London. Published in the Lancet (June 3, 2021):

|
Scooped by
Juan Lama
|
The coronavirus variant discovered in South Africa can "break through" Pfizer/BioNTech's COVID-19 vaccine to some extent, a real-world data study in Israel found, though its prevalence in the country is low and the research has not been peer reviewed. The study, released on Saturday, compared almost 400 people who had tested positive for COVID-19, 14 days or more after they received one or two doses of the vaccine, against the same number of unvaccinated patients with the disease. It matched age and gender, among other characteristics. The South African variant, B.1.351, was found to make up about 1% of all the COVID-19 cases across all the people studied, according to the study by Tel Aviv University and Israel’s largest healthcare provider, Clalit. But among patients who had received two doses of the vaccine, the variant’s prevalence rate was eight times higher than those unvaccinated - 5.4% versus 0.7%. This suggests the vaccine is less effective against the South African variant, compared with the original coronavirus and a variant first identified in Britain that has come to comprise nearly all COVID-19 cases in Israel, the researchers said. “We found a disproportionately higher rate of the South African variant among people vaccinated with a second dose, compared to the unvaccinated group. This means that the South African variant is able, to some extent, to break through the vaccine’s protection,” said Tel Aviv University’s Adi Stern. The researchers cautioned, though, that the study only had a small sample size of people infected with the South African variant because of its rarity in Israel. They also said the research was not intended to deduce overall vaccine effectiveness against any variant, since it only looked at people who had already tested positive for COVID-19, not at overall infection rates. Pfizer and BioNTech could not be immediately reached for comment outside business hours. The companies said on April 1 that their vaccine was around 91% effective at preventing COVID-19, citing updated trial data that included participants inoculated for up to six months. In respect to the South African variant, they said that among a group of 800 study volunteers in South Africa, where B.1.351 is widespread, there were nine cases of COVID-19, all of which occurred among participants who got the placebo. Of those nine cases, six were among individuals infected with the South African variant. Some previous studies have indicated that the Pfizer/BioNTech shot was less potent against the B.1.351 variant than against other variants of the coronavirus, but still offered a robust defence. While the results of the study may cause concern, the low prevalence of the South African strain was encouraging, according to Stern. “Even if the South African variant does break through the vaccine’s protection, it has not spread widely through the population,” said Stern, adding that the British variant may be “blocking” the spread of the South African strain. Almost 53% of Israel’s 9.3 million population has received both Pfizer doses. Israel has largely reopened its economy in recent weeks while the pandemic appears to be receding, with infection rates, severe illness and hospitalizations dropping sharply. About a third of Israelis are below the age of 16, which means they are still not eligible for the shot. Findings available in medRxiv (April 9, 2021): https://doi.org/10.1101/2021.04.06.21254882

|
Scooped by
Juan Lama
|
- Pfizer's and Moderna's shots were at least 10 times less effective against variant in a new study.
- Researchers tested the vaccines on a variant first found in South Africa, which is now in 20 states.
- A mutation on the variant called E484K appeared to be a "major contributor," the study authors said.
COVID-19 vaccines from Moderna and Pfizer-BioNTech appear significantly less effective against the coronavirus variant first found in South Africa, a lab study has suggested. The percentage of protective antibodies that neutralized the variant — called B.1.351, which has been recorded in 20 US states — was 12.4 fold lower for Moderna's COVID-19 shot than against the original coronavirus, and 10.3 fold lower for Pfizer's, the study authors said. This was a bigger drop than in previous lab studies testing the vaccines against manufactured forms of the variant, they said. For this study, the researchers used real forms of the variant taken from people who had caught the virus. Both the Pfizer and Moderna vaccines have been authorized for emergency use in the US. B.1.351 was first detected in South Africa in October 2020. It has since spread to 42 countries, including to the US, where it is circulating in at least 20 states, including California and Texas, the Centers for Disease Control and Prevention said. There are 81 reported cases of B.1.351 in the US overall, the CDC said. The researchers found that the antibody activity from both vaccines was "essentially unchanged" against the variant first found in the UK, B.1.1.7. There are 3,037 reported cases in the US of B.1.1.7, the CDC said, and experts believe it will soon become the dominant strain in the US. The scientists, from Columbia University, also tested lab-made viruses that had certain mutations. They said that one specific mutation, E484K, appeared to be a "major contributor" to the B.1.351 variant's ability to evade the antibody response. E484K is not usually present in B.1.1.7, the variant first found in the UK. The study has been accepted by science journal Nature but not yet published. Taking samples from the real world In the experiment, scientists took 10 blood samples from people who had received two doses of Pfizer's vaccine, 28 days after their second dose, and 12 samples from those who had received two doses of Moderna's vaccine, 43 days after their second dose. They then compared how well antibodies in the blood samples "neutralized" the original coronavirus, compared to real-life B.1.1.7 and B.1.351 coronavirus variants. The sample size was small, and the antibody response is just one aspect of the immune response, so it remains unclear how well the vaccines work against the variant first found in South Africa in real life. Pfizer has conducted petri-dish tests before that showed a less potent antibody response against a lab-made coronavirus variant that mimicked the variant first found in South Africa. It was not the exact B.1.351 variant. The study, published as correspondence to the New England Journal of Medicine February 17 and updated March 8, suggested the vaccine would work against the variant. It also showed that the antibody response from Pfizer's shot held up against the variant first found in Brazil, P.1, that has a similar set of mutations to B.1.351. Moderna ran similar tests and said that its vaccine held up well against the mutations found in B.1.1.7, the variant first found in the UK, but less well against the mutations found in B.1.351, the variant first identified in South Africa. Again, it used lab-made variants. Both companies said in January that they were developing booster shots specifically to tackle the B.1.351 variant. Neither of the vaccines has been properly tested against the variant first found in South Africa in the real world. In Israel, Pfizer's vaccine has been shown to be highly effective against the B.1.1.7 variant, first found in the UK. About 80% of Israelis with COVID-19 are infected with B.1.1.7. The COVID-19 vaccine from Johnson & Johnson was less effective in the clinical trials that took place in South Africa. Original Findings to Be Published in Nature (March 8, 2021):
|



 Your new post is loading...
Your new post is loading...