Your new post is loading...

|
Scooped by
Juan Lama
|
On September 1, 2022, the Moderna and Pfizer–BioNTech bivalent vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) containing equal amounts of spike messenger RNA from the ancestral and omicron BA.4–BA.5 subvariants replaced their monovalent counterparts as booster doses for persons who are 12 years of age or older in the United States. We previously reported surveillance data from North Carolina on the effectiveness of these two bivalent boosters against coronavirus disease 2019 (Covid-19) during the first 3 months after deployment (September 1 to December 8, 2022); the BA.4–BA.5 subvariants were predominant during the first 2.5 months of this period.1 Here, we present two additional months of data that were obtained during a period when the omicron BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants had become predominant to show the durability of protection conferred by these two bivalent boosters against a wider range of clinical outcomes than were included in our previous report. The data sources and study design have been described previously,1-3 and updated information is provided in the Methods section of the Supplementary Appendix, available with the full text of this letter at NEJM.org. The current study used data regarding booster doses and clinical outcomes from September 1, 2022, to February 10, 2023, for all North Carolina residents who were 12 years of age or older. During this period, a total of 6,306,311 residents were eligible to receive bivalent boosters; of these residents, 1,279,802 received the injections. A total of 19,462 of the 154,581 SARS-CoV-2 infections, 253 of the 2208 Covid-19–related hospitalizations, and 79 of the 867 Covid-19–related deaths occurred after receipt of the bivalent booster (Table S1 in the Supplementary Appendix). We considered four outcome measures: infection, severe infection resulting in hospitalization, severe infection resulting in hospitalization or death, and severe infection resulting in death. We fit the Cox regression model with a time-varying hazard ratio for severe infection and fit the proportional-rates model with a time-varying rate ratio for recurrent infection for each additional booster dose that was received (i.e., first booster vs. primary vaccination, second booster vs. first booster, or third booster vs. second booster); all measures were adjusted for the baseline characteristics shown in Table S1. We estimated the booster effectiveness on a particular day as 1 minus the hazard ratio or rate ratio on that day multiplied by 100%. Effectiveness against severe infection resulting in hospitalization was slightly lower, and effectiveness against infection was much lower. The effectiveness against severe infection resulting in death was the highest despite uncertainty because of the small number of events. We also analyzed the data separately for participants who received bivalent boosters before November 1, 2022 (when the BA.4–BA.5 subvariants were predominant) and after November 1, 2022 (when the BQ.1–BQ.1.1 subvariants were more prevalent and then were gradually replaced by the XBB–XBB.1.5 subvariants). The results are shown in the right column of Figure 1 and in Tables S3 and S4. The effectiveness was broadly similar between the two booster cohorts. Finally, we performed subgroup analyses according to the participant’s age and previous infection status and according to the manufacturers of the bivalent vaccine and the previous vaccine. Effectiveness against infection was higher for the Moderna bivalent vaccine than for the Pfizer–BioNTech bivalent vaccine and higher among previously infected participants than among those with no previous infection (Fig. S1). The two types of bivalent boosters were associated with an additional reduction in the incidence of omicron infection among participants who had previously been vaccinated or boosted. Although the two bivalent vaccines were designed to target the BA.4–BA.5 subvariants, they were also associated with a lower risk of infection or severe infection with the BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants. The effectiveness was higher against hospitalization and death than against infection and waned gradually from its peak over time. Published in NEJM (April 12, 2023): https://doi.org/10.1056/NEJMc2302462

|
Scooped by
Juan Lama
|
Germany's BioNTech , Pfizer's partner in COVID vaccines, said the two companies would start tests on humans of next-generation shots that protect against a wide variety of coronaviruses in the second half of the year. Their experimental work on shots that go beyond the current approach include T-cell-enhancing shots, designed to primarily protect against severe disease if the virus becomes more dangerous, and pan-coronavirus shots that protect against the broader family of viruses and its mutations. In presentation slides posted on BioNTech's website for its investor day, the German biotech firm said its aim was to "provide durable variant protection". The two partners, makers of the Western world's most widely used COVID-19 shot, are currently discussing with regulators enhanced versions of their established shot to better protect against the Omicron variant and its sublineages. read more The virus' persistent mutation into new variants that more easily evade vaccine protection, as well as waning human immune memory, have added urgency to the search by companies, governments and health bodies for more reliable tools of protection. As part of a push to further boost its infectious disease business, BioNTech said it was independently working on precision antibiotics that kill superbugs that have grown resistant to currently available anti-infectives. BioNTech, which did not say when trials could begin, is leaning on the technology of PhagoMed, which it acquired in October last year. The Vienna-based antibiotics developer has done work on enzymes, made by bacteria-killing viruses, that break through the bacterial cell wall. Drug-resistant infections are on the rise, driven by antibiotic overuse and leaks into the environment in antibiotics production. Public health researchers put the combined number of people dying per year from antibiotic-resistant infections in the United States and the European Union at close to 70,000. read more

|
Scooped by
Juan Lama
|
Pfizer and BioNTech have begun a clinical trial for their Omicron-specific Covid-19 vaccine candidate, they announced in a news release on Tuesday. The study will evaluate the vaccine for safety, tolerability and the level of immune response, as both a primary series and a booster dose, in up to 1,420 healthy adults ages 18 to 55. The study is broken up into three groups: Participants in the first cohort have received two doses of the current Pfizer Covid-19 vaccine at least 90 to 180 days before the study. They will receive one or two doses of the Omicron-specific vaccine. Participants in the second cohort have received three doses of the current Pfizer Covid-19 vaccine at least 90 to 180 days prior to the study. They will receive one dose of the current Pfizer Covid-19 vaccine or the Omicron-specific vaccine. Participants in the third cohort have not received any Covid-19 vaccine. They will receive three doses of the Omicron-specific vaccine. The Omicron-specific vaccine will be administered as a 30-microgram dose, the same as the current vaccine. "While current research and real-world data show that boosters continue to provide a high level of protection against severe disease and hospitalization with Omicron, we recognize the need to be prepared in the event this protection wanes over time and to potentially help address Omicron and new variants in the future," Pfizer Senior Vice President and Head of Vaccine Research and Development Kathrin Jansen said in the release. Pfizer CEO Albert Bourla said last month that if a new vaccine is needed for the Omicron coronavirus variant, the company will have one in March. However, a Pfizer spokesperson confirmed that the company has already begun to manufacture this vaccine. "In the wake of Omicron, we are proactively investigating and manufacturing at risk an Omicron-based vaccine should it be needed, but we of course need to have results and discussions with health authorities as well as approvals before it would be deployed," the spokesperson told CNN. Expected vaccine production will not be affected if the companies need to pivot to the new vaccine, they said. "The companies have previously announced that they expect to produce four billion doses of the Pfizer-BioNTech COVID-19 Vaccine in 2022, and this capacity is not expected to change if an adapted vaccine is required." However, the companies also emphasized that people who have received booster doses of the current vaccine "maintain a high level of protection against Omicron, particularly against severe disease and hospitalizations." A new preprint lab study suggests that antibodies against the Omicron coronavirus variant remain robust four months after a third dose of the Pfizer/BioNTech vaccine. "Additional real world effectiveness data and laboratory investigations will further inform the duration of protection, potential need for an additional dose at a later time, and whether an Omicron modified vaccine is required," said the study from researchers at the University of Texas Medical Branch, Pfizer and BioNTech. Pfizer Press Release (Jan. 25, 2022):

|
Scooped by
Juan Lama
|
Couple who pioneered jab given to millions around world tell how pharmaceutical giant wrongly assumed outbreak would be quickly contained. Pfizer initially turned down the offer of developing a coronavirus vaccine because its executives thought the virus would be rapidly contained. Dr Ugur Sahin and his wife, Dr Özlem Türeci, the founders of BioNTech, were told "guys, this is not going to work” by the pharmaceutical giant as the virus was starting to sweep the globe in January 2020. The mRNA technology, which has proved so crucial to the vaccine breakthroughs, was, at the time, also considered too experimental by Dr Phil Dormitzer, Pfizer’s vice-president and chief scientific officer for viral vaccines. “My working assumption was that it [Covid-19] would be controlled” like the Sars and Mers outbreaks, Dr Dormitzer admits. The initial rejection, revealed in a new book, came just days after the Turkish-born couple decided to dedicate BionNTech to creating an mRNA based Covid jab, effectively gambling the business on something that had never been done before. Their company is now worth US$85 billion. Yet Drs Sahin and Türeci remain close to Pfizer and Dr Dormitzer, or “Phil” as they know him. Dr Sahin had a detailed image in his mind of how the pandemic would unfold but also thought the Pfizer man’s assessment “completely rational”. “After the phone call with Phil, I just thought for a second and said ‘we will call him again in a few weeks,’” Dr Sahin told The Telegraph. The couple thought it only a “matter of time” before the drugs giant changed its mind – and they were right. A deal was announced between the two companies a month later. Next week, the Government is expected to announce a "booster" campaign for higher risk groups, which the Pfizer jab is expected to be at the forefront of. Boris Johnson will also announce the repeal of a series of measures from the Coronavirus Act which are now deemed unnecessary. These include powers to close-down sectors of the economy, such as business premises, or apply restrictions to events and gatherings. Husband and wife scientists’ vaccine gamble was a shot in the arm for millions BioNTech may have created the world’s leading coronavirus vaccine but most of its staff are still working remotely, whether double or triple jabbed – and that includes its founders. “I’m here most of the time but the company is about 250 metres from here, so sometimes I'm just watching to see if everyone is working”, says Dr Ugur Şahin over a Zoom link from a temporary location. “It’s to avoid too much contact”, interjects his wife and business partner, Dr Özlem Türeci, perhaps sensing Dr Sahin’s joke with British journalists may go horribly wrong. “To let the people on the campus do their work in the labs and the manufacturing”. This dynamic - Dr Sahin enthusing, Dr Türeci copper-bottoming - may help explain how an obscure biotech firm from an unfashionable part of Germany ended up vaccinating so much of the world. So far, roughly 1.4 billion doses of their revolutionary jab - each containing billions of nanoparticles of synthetic RNA code - have been shipped to more than 120 countries. Only the Chinese vaccine manufacturers have distributed more. Their story is recounted in a new book, The Vaccine, written by journalist Joe Miller with the couples’ cooperation, to be released this week. It tells how the couple, who emigrated to Germany from Turkey as small children and met on a cancer ward as young doctors, built not one billion-dollar biotech company but two. And how they gambled everything to pivot BioNTech to focus exclusively on a Covid vaccine in early 2020. It’s proved a phenomenal success but when Dr Sahin initially called Pfizer to see if it wanted to be involved, the answer was a firm “no”. “Guys, this is not going to work”, he was told by Dr Phil Dormitzer, Pfizer’s vice-president and chief scientific officer for vaccines. Mr Dormitzer had been involved with discussions about whether to create vaccines for Mers and Sars, only to see the pathogens quickly contained, and thought the same would be true of Sars-Cov-2. “My working assumption was that it would be controlled”, he later confirmed to Miller. None of this is recounted by the scientists with any hint of ego or malice. Dr Sahin is 56 but he exudes the energy of a 18 year old and does not seem to have a corporate bone in his body. He talks to us in a scruffy T-shirt with a hippy-ish thong around his neck. Dr Türeci is slightly more formal and has a doctor’s air of benevolent patience. “After the phone call with Phil, I just thought for a second and said ‘we will call him again in a few weeks,’” Dr Sahin recalls. It was the publication of a single article by Chinese academics in the Lancet on January 24 2020 that convinced the couple that a pandemic was coming and that they should act, no matter what the corporate risk. The article provided the first strong evidence of human-to-human transmission but, for Dr Sahin, there was something more: a seven-year-old girl mentioned in the study had tested positive for the virus without first displaying symptoms....

|
Scooped by
Juan Lama
|
The decision will set off a cascade of vaccine requirements by hospitals, colleges, corporations, and some state and local governments. The Food and Drug Administration on Monday granted full approval to Pfizer-BioNTech’s coronavirus vaccine for people 16 and up, making it the first to move beyond emergency use status in the United States. The decision will set off a cascade of vaccine requirements by hospitals, colleges, corporations and other organizations. United Airlines recently announced that its employees will be required to show proof of vaccination within five weeks of regulatory approval. Oregon has adopted a similar requirement for all state workers, as have a host of universities in states from Louisiana to Minnesota. The Pentagon has said it would mandate the shots for the country’s 1.3 million active-duty troops once the Pfizer approval came through. The approval comes as the nation’s fight against the pandemic has intensified again, with the highly infectious Delta variant hurting the progress that the country had made over the first half of the year. The Biden administration hopes the development will motivate at least some of the roughly 85 million unvaccinated Americans who are eligible for shots to get them. “While millions of people have already safely received Covid-19 vaccines, we recognize that for some, the F.D.A. approval of a vaccine may now instill additional confidence to get vaccinated,” Dr. Janet Woodcock, the acting F.D.A. commissioner, said in a statement. “Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.” Pfizer said it presented the F.D.A. with data from 44,000 clinical trial participants in United States, the European Union, Turkey, South Africa and South America. The company said the data showed the vaccine was 91 percent effective in preventing infection — a slight drop from the 95 percent efficacy rate that the data showed when the F.D.A. decided to authorize the vaccine for emergency use in December. Pfizer said the decrease reflected the fact that researchers had more time to catch people who became infected. A recent poll by the Kaiser Family Foundation, which has been tracking public attitudes during the pandemic, found that three of every 10 unvaccinated people said that they would be more likely to get vaccinated with a shot that had been fully approved. The Pfizer-BioNTech vaccine will continue to be authorized for emergency use for children ages 12 to 15 while Pfizer collects the necessary data required for full approval. A decision on whether to authorize the vaccine for children younger than 12 could be at least several months away. So far, more than 92 million Americans — 54 percent of those fully inoculated — have gotten Pfizer shots. Most of the rest received Moderna’s vaccine. Dr. Peter Marks, the F.D.A.’s top vaccine regulator, said that the Pfizer vaccine’s licensure followed a rigorous review of hundreds of thousands of pages of data and included inspections of the factories where the vaccine is produced. “The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.,” he said. The F.D.A. is in the midst of a decision-making marathon related to coronavirus vaccines. The next major one looming for regulators is whether or not to authorize booster shots. The Biden administration said last week that pending the agency’s clearance, it will offer third shots to adults who got the Pfizer and Moderna vaccines eight months after their second injection, starting Sept. 20. Federal health officials said that both Pfizer-BioNTech and Moderna’s vaccines, which rely on similar technology, wane in potency over time. That trend, they said, is converging with the rise of the particularly dangerous Delta variant, making those who completed their vaccinations at the start of the year increasingly vulnerable to infection. Some health experts have challenged the decision to recommend booster shots as premature, saying the data shows that the vaccines are holding up well against severe disease and hospitalization, including against the Delta variant. Boosters would only be warranted if the vaccines were failing to prevent hospitalizations with Covid-19, some of those experts have said. Regulators are still reviewing Moderna’s application for full approval of its vaccine. That decision could take several weeks. Johnson & Johnson is expected to apply soon for full approval. Sharon LaFraniere is an investigative reporter. She was part of a team that won a Pulitzer Prize in 2018 for national reporting on Donald Trump’s connections with Russia. @SharonLNYT Noah Weiland is a reporter in the Washington bureau, covering health care. He was raised in East Lansing, Mich., and graduated from the University of Chicago. @noahweiland

|
Scooped by
Juan Lama
|
People who got mixed doses of coronavirus vaccines -- receiving a different vaccine type as a second dose than the first dose -- appear to be more likely to experience mild side effects such as fever, chills, fatigue or headache, researchers in the UK reported Wednesday. But the side effects following mix-and-match vaccinations were short-lived and there were no other safety concerns, the researchers reported in the Lancet medical journal. "These are the type of reactions you do expect with vaccine," Dr. Matthew Snape, an associate professor of pediatrics and vaccinology at the University of Oxford and chief investigator on the trial, said during a media briefing. "They are more or less the same types of reactions that you're seeing with the standard schedules. It's just that they're occurring more frequently, and we're seeing both more frequent both in mild and moderate symptoms -- but they resolved quickly," Snape said. Overall, "it's a really intriguing finding," he said, "and it's not something necessarily we were expecting -- to see such a consistent signal." It's something to keep an eye out for when giving mixed doses, the researchers said. "One of the things it's telling us is that, for example, you wouldn't want to immunize a ward full of nurses on the same day with a mixed schedule," Snape said. "Because you may have higher rates of absenteeism in the next day." The mix-and-match trial The new research included 830 volunteers 50 and older who were randomly assigned to four different vaccine schedules involving the Oxford/AstraZeneca and Pfizer/BioNTech vaccines, with first and second doses given 28 days apart. They either got the AstraZeneca vaccine as both doses; AstraZeneca as a first dose and Pfizer as a second dose; the Pfizer vaccine as both doses; or the Pfizer vaccine as a first dose and AstraZeneca as a second dose. The researchers found that people who got different vaccines had more side effects following the second dose, with feverishness reported by 34% of those who received the AstraZeneca vaccine first and Pfizer vaccine second, compared with 10% of those given the AstraZeneca vaccine for both doses. Fever was reported by 41% of the people who received the Pfizer vaccine first and AstraZeneca vaccine second, compared with 21% of the volunteers given the Pfizer vaccine for both doses. "Similar increases were observed for chills, fatigue, headache, joint pain, malaise, and muscle ache," the researchers wrote. They noted that people could take acetaminophen -- sold under brand names such as Tylenol -- to ease the side-effects. There were no hospitalizations due to the symptoms and most of the increased reactions were seen within 48 hours after immunization, the researchers found. They noted that they did not see evidence of a rare blood clotting syndrome that's been linked with the AstraZeneca and Johnson & Johnson vaccines in any of the volunteers within a week after the second dose. The researchers also noted that their findings are based on initial data and there are now ongoing studies testing mixed administration of vaccines made by Moderna and Novavax. More research is also needed to evaluate immune responses following different types of schedules, and whether increased side effects suggest that schedules using different types of vaccines elicit strong immune responses. "We do think reactions often relate to the stimulating of the innate immune response," Snape said. "Whether or not this will relate to actually an improved immune response we don't know yet. We'll be finding out those results in a few weeks time. Seeking 'greater insight' The US Centers for Disease Control and Prevention and the World Health Organization do not currently recommend interchanging coronavirus vaccines -- but the CDC noted in January that its guidance may be updated as new information and new types of vaccines become available. There may be advantages to having more flexible vaccine schedules using different vaccine types as first and second doses, Dr. Jonathan Van-Tam, England's deputy chief medical officer, said in a statement in February when the new research first began. "Given the inevitable challenges of immunizing large numbers of the population against COVID-19 and potential global supply constraints, there are definite advantages to having data that could support a more flexible immunization program, if needed and if approved by the medicines regulator," Van-Tam said at the time. "It is also even possible that by combining vaccines, the immune response could be enhanced giving even higher antibody levels that last longer; unless this is evaluated in a clinical trial we just won't know," he said. "This study will give us greater insight into how we can use vaccines to stay on top of this nasty disease." See The Lancet publication (May 12):

|
Scooped by
Juan Lama
|
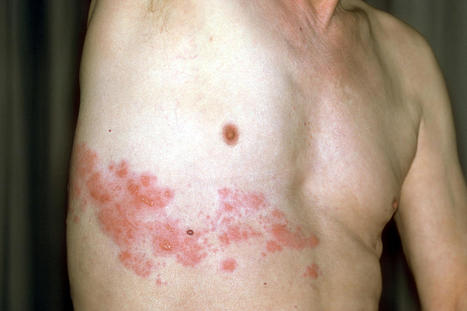
Herpes infections may be a side effect of the COVID-19 vaccine, experts have revealed. Scientists in Israel identified six cases in a new study of patients developing a skin rash known as herpes zoster after receiving the Pfizer vaccine, according to a study in the Rheumatology journal. Herpes zoster starts off as a small, itchy skin rash, but if left untreated, it could cause nerve damage and pain, the Jerusalem Post reported. This can include a prolonged burning sensation on the skin even after the rash disappears. Researchers from Tel Aviv Sourasky Medical Center and Carmel Medical Center in Haifa found those with autoimmune inflammatory rheumatic diseases had a higher risk of developing the herpes. Out of 491 patients, six people or 1.2 percent experienced the infection, researchers said. The six patients all have mild cases of autoimmune inflammatory rheumatic diseases and were young, though the infection is generally more common in those over the age of 50. “That is why we reported on it,” Dr. Victoria Furer, the lead author, told the outlet. Five of them developed herpes zoster after the first dose and the sixth got it after the second. But it’s still unclear whether the vaccine caused the cases of herpes zoster. “We cannot say the vaccine is the cause at this point,” Furer told the outlet. “We can say it might be a trigger in some patients.” “We should not scare people,” she told the Jerusalem Post. “The overall message is to get vaccinated. It is just important to be aware.” Original research published in Rheumathology (April 12, 2021): https://doi.org/10.1093/rheumatology/keab345

|
Scooped by
Juan Lama
|
Pfizer Inc and BioNTech said on Thursday their COVID-19 vaccine is around 91% effective at preventing the disease, citing updated trial data that included participants inoculated for up to six months. The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said.The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said. While the new overall efficacy rate of 91.3% is lower than the 95% originally reported in November for its 44,000-person trial, a number of variants have become more prevalent around the world since then. Pfizer’s Chief Executive Officer Albert Bourla said the updated result, which includes data on more than 12,000 people fully inoculated for at least six months, positions the drugmakers to submit for full U.S. regulatory approval. The vaccine is currently authorized on an emergency basis by the U.S. Food and Drug Administration. The trial data “provide the first clinical results that a vaccine can effectively protect against currently circulating variants, a critical factor to reach herd immunity and end this pandemic for the global population,” Ugur Sahin, chief executive officer at BioNTech, said in a statement. Experts fear new variants of COVID-19 from South Africa and Brazil may be resistant to existing vaccines and treatment. More than 300 cases of the South African variant have been detected in more than 25 U.S. states and jurisdictions, according to federal data. Lab tests have previously indicated that BioNTech’s vaccine was less potent but still offered a robust defense against the B1351 variant that first emerged in South Africa. Still, BioNTech reiterated this week there would likely be a future need for booster shots that specifically address new variants and that the group was preparing to upgrade its vaccine when needed. BioNTech has said that it started testing a modified vaccine version against the South African mutant in March for early indications on safety and efficacy but a product for later market launch would require yet another redesign and more tests. The updated trial data would not prompt the company to change that development strategy, a BioNTech spokeswoman said. Pfizer's Press Release (April 1, 2021): https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-confirm-high-efficacy-and-no-serious

|
Scooped by
Juan Lama
|
- Pfizer's and Moderna's shots were at least 10 times less effective against variant in a new study.
- Researchers tested the vaccines on a variant first found in South Africa, which is now in 20 states.
- A mutation on the variant called E484K appeared to be a "major contributor," the study authors said.
COVID-19 vaccines from Moderna and Pfizer-BioNTech appear significantly less effective against the coronavirus variant first found in South Africa, a lab study has suggested. The percentage of protective antibodies that neutralized the variant — called B.1.351, which has been recorded in 20 US states — was 12.4 fold lower for Moderna's COVID-19 shot than against the original coronavirus, and 10.3 fold lower for Pfizer's, the study authors said. This was a bigger drop than in previous lab studies testing the vaccines against manufactured forms of the variant, they said. For this study, the researchers used real forms of the variant taken from people who had caught the virus. Both the Pfizer and Moderna vaccines have been authorized for emergency use in the US. B.1.351 was first detected in South Africa in October 2020. It has since spread to 42 countries, including to the US, where it is circulating in at least 20 states, including California and Texas, the Centers for Disease Control and Prevention said. There are 81 reported cases of B.1.351 in the US overall, the CDC said. The researchers found that the antibody activity from both vaccines was "essentially unchanged" against the variant first found in the UK, B.1.1.7. There are 3,037 reported cases in the US of B.1.1.7, the CDC said, and experts believe it will soon become the dominant strain in the US. The scientists, from Columbia University, also tested lab-made viruses that had certain mutations. They said that one specific mutation, E484K, appeared to be a "major contributor" to the B.1.351 variant's ability to evade the antibody response. E484K is not usually present in B.1.1.7, the variant first found in the UK. The study has been accepted by science journal Nature but not yet published. Taking samples from the real world In the experiment, scientists took 10 blood samples from people who had received two doses of Pfizer's vaccine, 28 days after their second dose, and 12 samples from those who had received two doses of Moderna's vaccine, 43 days after their second dose. They then compared how well antibodies in the blood samples "neutralized" the original coronavirus, compared to real-life B.1.1.7 and B.1.351 coronavirus variants. The sample size was small, and the antibody response is just one aspect of the immune response, so it remains unclear how well the vaccines work against the variant first found in South Africa in real life. Pfizer has conducted petri-dish tests before that showed a less potent antibody response against a lab-made coronavirus variant that mimicked the variant first found in South Africa. It was not the exact B.1.351 variant. The study, published as correspondence to the New England Journal of Medicine February 17 and updated March 8, suggested the vaccine would work against the variant. It also showed that the antibody response from Pfizer's shot held up against the variant first found in Brazil, P.1, that has a similar set of mutations to B.1.351. Moderna ran similar tests and said that its vaccine held up well against the mutations found in B.1.1.7, the variant first found in the UK, but less well against the mutations found in B.1.351, the variant first identified in South Africa. Again, it used lab-made variants. Both companies said in January that they were developing booster shots specifically to tackle the B.1.351 variant. Neither of the vaccines has been properly tested against the variant first found in South Africa in the real world. In Israel, Pfizer's vaccine has been shown to be highly effective against the B.1.1.7 variant, first found in the UK. About 80% of Israelis with COVID-19 are infected with B.1.1.7. The COVID-19 vaccine from Johnson & Johnson was less effective in the clinical trials that took place in South Africa. Original Findings to Be Published in Nature (March 8, 2021):

|
Scooped by
Juan Lama
|
Asymptomatic coronavirus infections were four times less frequent in health-care workers who had received a single dose of a prominent COVID-19 vaccine than in their unvaccinated counterparts. Michael Weekes at the University of Cambridge, UK, and his colleagues analysed the results of almost 8,900 SARS-CoV-2 tests taken by UK health-care workers without symptoms of COVID-19 (M. Weekes et al. Preprint at Authorea https://doi.org/fxkd; 2021). Study participants who were tested at least 12 days after receiving one dose of the vaccine developed by Pfizer of New York City and BioNTech of Mainz, Germany, had an infection rate of only 0.2%. By contrast, unvaccinated participants had an infection rate of 0.8%. The team also noted that participants who showed evidence of SARS-CoV-2 infection well after vaccination tended to have lower levels of the coronavirus in their bodies than did those who were infected and unvaccinated, although the result did not reach statistical significance. If corroborated, this would suggest that the few vaccinated health-care workers who do have an asymptomatic infection are less likely to infect other people than are unvaccinated workers who become infected. The findings have not yet been peer reviewed.

|
Scooped by
Juan Lama
|
The University of Oxford will lead the first trial exploring whether different Covid-19 vaccines can be used interchangeably, the University’s vaccine group said Thursday, an effort that could make vaccination programs more flexible and even provide better protection against the disease. The study, run by the U.K. National Immunisation Schedule Evaluation Consortium, will recruit over 800 people over the age of 50 to evaluate the feasibility of using one vaccine for the first “prime” shot and different one for the second “booster” shot. At first, the trial will use the Oxford-AstraZeneca and Pfizer-BioNTech vaccines, both already cleared for emergency use in the U.K., with others potentially being added at a later date. Britain’s Deputy Chief Medical Officer, Jonathan Van-Tam, said the trial would offer "greater insight" into the use of vaccines against Covid-19, and provide data that could support a “more flexible immunization programme" in light of global supply chain issues. "It is also even possible that by combining vaccines, the immune response could be enhanced giving even higher antibody levels that last longer," Van-Tam added. The study will also evaluate the effectiveness of different vaccine combinations when the booster shot is given after different intervals: one after four weeks and another after 12, which is the U.K.’s current approach in a bid to give as many people some form of immunity as quickly as possible. If the results are promising, the U.K. government said it would consider altering its national vaccination strategy. Professor Matthew Snape, Oxford’s chief investigator on the trial, said: “This is a tremendously exciting study that will provide information vital to the roll out of vaccines in the U.K. and globally… If we do show that these vaccines can be used interchangeably in the same schedule this will greatly increase the flexibility of vaccine delivery, and could provide clues as to how to increase the breadth of protection against new virus strains.” Some vaccines work better if a different vaccine is used for the booster shot, which is known as heterologous boosting. Russia’s Sputnik V vaccine, which early studies indicate to be 92% effective at preventing Covid-19, makes use of the principle. It uses of a different modified virus in each shot to carry the immunity-conveying instructions into the body.

|
Scooped by
Juan Lama
|
Pfizer Inc. and BioNTech SE built the case that their Covid-19 vaccine will protect against the new variant of the coronavirus that emerged in the U.K. with results of another lab trial. Like previous work out of the University of Texas Medical Branch, the results published on Wednesday showed that antibodies in the blood of people who had been vaccinated were able to neutralize a version of the mutant virus that was created in the lab. The study was published on preprint server BioRxiv prior to peer review. Unlike the earlier study, which focused on one crucial mutation, the new research tested all 10 mutations located on the virus’s spike protein, which helps it bind to cells in the host. It’s a promising but not conclusive result, as scientists continue to closely monitor whether mutations in the virus may make it necessary to adjust the vaccines. Antibodies in the blood of 16 volunteers in a previous German trial of the vaccine were just as effective against the lab-created mutant strain as they were against the original virus. The result “makes it very unlikely that the U.K. variant viruses will escape” protection from the vaccine, wrote the research team, led by BioNTech Chief Executive Officer Ugur Sahin. The BioNTech team is nevertheless ready to adapt the vaccine if needed in the future, it said. That could become necessary to protect against other strains amid evidence another variant that emerged in South Africa may be harder to check. A separate study on that strain raised concern. Scientists found that half of the blood samples from a handful of patients who already had Covid-19 don’t have the antibodies needed to protect against the South African variant, which is spreading around the globe. The findings, from South Africa’s National Institute for Communicable Diseases, suggest that those individuals may no longer be protected from re-infection. In the other half, antibody levels were reduced and the risk of re-infection couldn’t be determined, according to the institute. The findings weren’t peer-reviewed and were based on a small sample size. Separately, a third study from a Rockefeller University team also underlined the importance of keeping a close watch on the effectiveness of vaccines against variants. The team tested mutations found in the variants first discovered in the U.K. and South Africa, as well as a third from Brazil, in blood samples from 20 volunteers who had gotten either the Pfizer-BioNTech vaccine or a similar shot from Moderna Inc. In their test, the donors’ blood samples weren’t quite as effective at neutralizing the variants. “Vaccines may need to be updated periodically to avoid potential loss of clinical efficacy,” the Rockefeller team wrote. Like the other studies, their work was presented in pre-print, before peer review. Preprint available at bioRxiv (Jan. 19, 2021): https://doi.org/10.1101/2021.01.18.426984

|
Scooped by
Juan Lama
|
LESS THAN TWO DOZEN OF the nearly 2 million people who received the first doses of Pfizer's coronavirus vaccine experienced anaphylaxis, a dangerously severe form of allergic reaction. An analysis Wednesday from the Centers for Disease Control and Prevention and Food and Drug Administration showed that from Dec. 14 to 23, there were 21 cases of anaphylaxis after administration of nearly 1.9 million first doses of the Pfizer-BioNTech COVID-19 vaccine. Nearly three-quarters of those reactions – 71% – occurred within 15 minutes of vaccination. Anaphylaxis is a life-threatening allergic reaction that the CDC says can occur after a vaccination, although rarely. It typically occurs within minutes to hours. The 21 cases of anaphylaxis were among 4,393 reports of adverse events following a dose of the Pfizer vaccination, according to the CDC. Among those instances, 175 reports were identified for further review as possible severe allergic reactions. Seventeen of the 21 people who experienced anaphylaxis had documented histories of allergies or allergic reactions, including seven who had a history of anaphylaxis. Among 20 people for which follow-up information was available, all had recovered or had been released home, according to the CDC. The 21 cases of anaphylaxis translate to a rate of 11.1 cases per 1 million doses. In a briefing on Wednesday, Dr. Nancy Messonnier, director of the CDC's National Center for Immunization and Respiratory Diseases, said the rate of anaphylaxis for flu vaccine is around 1.3 cases per 1 million doses. "I guess you could mathematically say that's 10 times the amount. But I think that misses the point because it's still exceedingly rare," said Messonier, who called the Pfizer vaccine "very safe." The "risk from COVID and poor outcomes from COVID is still more than their risk of a severe outcome from the vaccine." Of the remaining 175 reports of allergic reactions, 86 were deemed nonanaphylaxis allergic reactions, 61 were considered nonallergic adverse events and seven were still under investigation, according to the analysis. The COVID-19 vaccine developed by Pfizer and BioNTech received emergency use authorization in the U.S. last month and has been deemed 95% effective in preventing COVID-19, which has infected more than 21 million people in the country and killed more than 359,000. Many top politicians have publicly received the vaccine to instill public confidence in it, including Vice President Mike Pence and Vice President-elect Joe Biden. The CDC analysis states that mortality from COVID-19 in populations at high risk is "substantial, and treatment options are limited. Widespread vaccination against COVID-19 with highly effective vaccines represents an important tool in efforts to control the pandemic." Original Findings published by the CDC in MMWR (Jan. 6, 2021): http://dx.doi.org/10.15585/mmwr.mm7002e1
|

|
Scooped by
Juan Lama
|
The SARS-CoV-2 Omicron variant and its numerous sub-lineages have exhibited a striking ability to evade humoral immune responses induced by prior vaccination or infection. The Food and Drug Administration (FDA) has recently granted Emergency Use Authorizations (EUAs) to new bivalent formulations of the original Moderna and Pfizer mRNA SARS-CoV-2 vaccines that target both the ancestral strain as well as the Omicron BA.4/BA.5 variant. Despite their widespread use as a vaccine boost, little is known about the antibody responses induced in humans. Here, we collected sera from several clinical cohorts: individuals after three or four doses of the original monovalent mRNA vaccines, individuals receiving the new bivalent vaccines as a fourth dose, and individuals with BA.4/BA.5 breakthrough infection following mRNA vaccination. Using pseudovirus neutralization assays, these sera were tested for neutralization against an ancestral SARS-CoV-2 strain, several Omicron sub-lineages, and several related sarbecoviruses. At ~3-5 weeks post booster shot, individuals who received a fourth vaccine dose with a bivalent mRNA vaccine targeting BA.4/BA.5 had similar neutralizing antibody titers as those receiving a fourth monovalent mRNA vaccine against all SARS-CoV-2 variants tested, including BA.4/BA.5. Those who received a fourth monovalent vaccine dose had a slightly higher neutralizing antibody titers than those who received the bivalent vaccine against three related sarbecoviruses: SARS-CoV, GD-Pangolin, and WIV1. When given as a fourth dose, a bivalent mRNA vaccine targeting Omicron BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not induce superior neutralizing antibody responses in humans, at the time period tested, compared to the original monovalent vaccine formulation. Preprint available in bioRxiv (Oct. 24, 2022): https://www.biorxiv.org/content/10.1101/2022.10.22.513349v1 https://doi.org/10.1101/2022.10.22.513349

|
Scooped by
Juan Lama
|
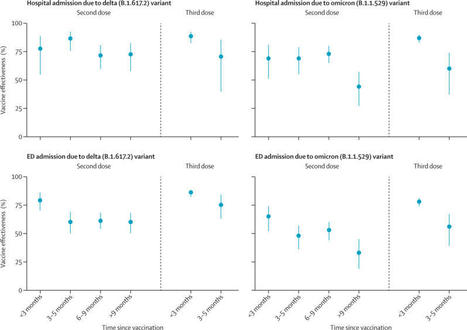
Background The duration of protection against the omicron (B.1.1.529) variant for current COVID-19 vaccines is not well characterised. Vaccine-specific estimates are especially needed. We aimed to evaluate the effectiveness and durability of two and three doses of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine against hospital and emergency department admissions due to the delta (B.1.617.2) and omicron variants. Methods In this case–control study with a test-negative design, we analysed electronic health records of members of Kaiser Permanente Southern California (KPSC), a large integrated health system in California, USA, from Dec 1, 2021, to Feb 6, 2022. Vaccine effectiveness was calculated in KPSC patients aged 18 years and older admitted to hospital or an emergency department (without a subsequent hospital admission) with a diagnosis of acute respiratory infection and tested for SARS-CoV-2 via PCR. Adjusted vaccine effectiveness was estimated with odds ratios from adjusted logistic regression models. This study is registered with ClinicalTrials.gov (NCT04848584). Findings Analyses were done for 11 123 hospital or emergency department admissions. In adjusted analyses, effectiveness of two doses of the BNT162b2 vaccine against the omicron variant was 41% (95% CI 21–55) against hospital admission and 31% (16–43) against emergency department admission at 9 months or longer after the second dose. After three doses, effectiveness of BNT162b2 against hospital admission due to the omicron variant was 85% (95% CI 80–89) at less than 3 months but fell to 55% (28–71) at 3 months or longer, although confidence intervals were wide for the latter estimate. Against emergency department admission, the effectiveness of three doses of BNT162b2 against the omicron variant was 77% (72–81) at less than 3 months but fell to 53% (36–66) at 3 months or longer. Trends in waning against SARS-CoV-2 outcomes due to the delta variant were generally similar, but with higher effectiveness estimates at each timepoint than those seen for the omicron variant. Interpretation Three doses of BNT162b2 conferred high protection against hospital and emergency department admission due to both the delta and omicron variants in the first 3 months after vaccination. However, 3 months after receipt of a third dose, waning was apparent against SARS-CoV-2 outcomes due to the omicron variant, including hospital admission. Additional doses of current, adapted, or novel COVD-19 vaccines might be needed to maintain high levels of protection against subsequent waves of SARS-CoV-2 caused by the omicron variant or future variants with similar escape potential. Published (April 22, 2022) in The Lancet Respiratory Medicine:

|
Scooped by
Juan Lama
|
Vaccinated kids aged 5 to 11 showed evidence of protection against the virus, the company said. The data must be reviewed by the F.D.A. before children can be inoculated. The Pfizer-BioNTech coronavirus vaccine has been shown to be safe and highly effective in young children aged 5 to 11 years, the companies announced early Monday morning. The news sets the stage for authorization of the vaccine for younger children, possibly before the end of October. The need is urgent: Children now account for more than one in five new cases, and the highly contagious Delta variant has sent more children into hospitals and intensive care units in the past few weeks than at any other time in the pandemic. Pfizer and BioNTech plan to apply to the Food and Drug Administration by the end of September for authorization to use the vaccine in these children. If the regulatory review goes as smoothly as it did for older children and adults — it took roughly a month — millions of elementary school students could begin to receive shots around Halloween. Trial results for children younger than 5 are not expected till the fourth quarter of this year at the earliest, according to Dr. Bill Gruber, a senior vice president at Pfizer and a pediatrician. Results from Moderna’s vaccine trials in children under 12 are also expected around that time, said Dr. Paul Burton, the company’s chief medical officer. Pfizer and BioNTech announced the results in a statement that did not include detailed data from the trial. The findings have not yet been peer-reviewed nor published in a scientific journal. But the new results dovetail with those seen in older children and in adults, experts said. “There’s going to be a huge number of parents who are going to heave a big sigh of relief when they hear this,” said Dr. Kristin Oliver, a pediatrician and vaccine expert at Mount Sinai Hospital in New York. “We’ve been waiting for these kids to be protected.” Before the vaccine can be authorized, F.D.A. scientists must carefully sift through the data, looking for side effects the company may have missed, which may slightly delay the process. Children have a much lower risk of Covid-19 than adults, even when exposed to the Delta variant. Still, some small number of infected children develop a life-threatening condition called multi-system inflammatory syndrome in children, or MIS-C. Still others may have lingering symptoms for months. Nearly 30,000 children were hospitalized for Covid in August; the least vaccinated states reported the highest rates. At Seattle Children’s hospital, about half of the children who are admitted for Covid are older than 12, according to Dr. Danielle Zerr, a pediatric infectious diseases expert at the hospital. “I’ve been dismayed at the fact that the sickest children in our hospital with acute Covid-19 or MIS-C are children who could have been vaccinated,” Dr. Zerr said. As ideological battles over masking and vaccine mandates play out in communities, the reopening of schools has fueled the surge. In Mississippi, among the states without a mask mandate, nearly 6,000 students tested positive for the virus in one week, and more than 30,000 students, teachers and staff had to be quarantined. One county in South Carolina — where mask mandates are banned — had to quarantine more than 2,000 students in one day. Remote learning is not an option in many districts, so the safety of some medically vulnerable children in many parts of the country has become subject to the actions of others. The trial results were greeted enthusiastically by many school administrators and teachers’ organizations, but are unlikely to lead to immediate policy changes. “This is one huge step toward beating Covid and returning to normalcy. I don’t think it changes the conversation around vaccine requirements for kids,” said Randi Weingarten, president of the American Federation of Teachers, a national union. Ms. Weingarten noted that parents and educators were still awaiting full F.D.A. approval of vaccines for children aged 12 to 15, and that mandates for adults did not come until months after the shots first became available.... Pfizer release (Sept. 20, 2021) available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-positive-topline-results

|
Scooped by
Juan Lama
|
BACKGROUND Preapproval trials showed that messenger RNA (mRNA)–based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had a good safety profile, yet these trials were subject to size and patient-mix limitations. An evaluation of the safety of the BNT162b2 mRNA vaccine with respect to a broad range of potential adverse events is needed. METHODS We used data from the largest health care organization in Israel to evaluate the safety of the BNT162b2 mRNA vaccine. For each potential adverse event, in a population of persons with no previous diagnosis of that event, we individually matched vaccinated persons to unvaccinated persons according to sociodemographic and clinical variables. Risk ratios and risk differences at 42 days after vaccination were derived with the use of the Kaplan–Meier estimator. To place these results in context, we performed a similar analysis involving SARS-CoV-2–infected persons matched to uninfected persons. The same adverse events were studied in the vaccination and SARS-CoV-2 infection analyses. RESULTS In the vaccination analysis, the vaccinated and control groups each included a mean of 884,828 persons. Vaccination was most strongly associated with an elevated risk of myocarditis (risk ratio, 3.24; 95% confidence interval [CI], 1.55 to 12.44; risk difference, 2.7 events per 100,000 persons; 95% CI, 1.0 to 4.6), lymphadenopathy (risk ratio, 2.43; 95% CI, 2.05 to 2.78; risk difference, 78.4 events per 100,000 persons; 95% CI, 64.1 to 89.3), appendicitis (risk ratio, 1.40; 95% CI, 1.02 to 2.01; risk difference, 5.0 events per 100,000 persons; 95% CI, 0.3 to 9.9), and herpes zoster infection (risk ratio, 1.43; 95% CI, 1.20 to 1.73; risk difference, 15.8 events per 100,000 persons; 95% CI, 8.2 to 24.2). SARS-CoV-2 infection was associated with a substantially increased risk of myocarditis (risk ratio, 18.28; 95% CI, 3.95 to 25.12; risk difference, 11.0 events per 100,000 persons; 95% CI, 5.6 to 15.8) and of additional serious adverse events, including pericarditis, arrhythmia, deep-vein thrombosis, pulmonary embolism, myocardial infarction, intracranial hemorrhage, and thrombocytopenia. CONCLUSIONS In this study in a nationwide mass vaccination setting, the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events examined. The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events was substantially increased after SARS-CoV-2 infection. (Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.) Published in New England J. Medicine (August 25, 2021): https://doi.org/10.1056/NEJMoa2110475

|
Scooped by
Juan Lama
|
The SARS-CoV-2 B.1.617.2 Variant of Concern (VOC), first detected in India, is now dominant in the UK, having rapidly displaced the B.1.1.7 strain that emerged in the UK with the second COVID-19 wave in late 2020. The efficacy of currently licensed COVID-19 vaccines against B.1.617.2 is unknown; although it possesses 12 mutations in its spike protein relative to the wildtype SARS-CoV-2 first detected in Wuhan, China, in December, 2019, B.1.617.2 lacks mutations at amino acid positions 501 or 484 in its ACE2 receptor-binding domain, commonly associated with VOCs (appendix p 2) or escape from neutralising antibodies (NAbs). To determine vaccine-induced NAb escape by B.1.617.2 and compare activity to previous strains with existing estimates for population-based vaccine efficacy, we carried out an initial analysis of the Legacy study, established in January, 2021, by University College London Hospital and the Francis Crick Institute in London, UK, to track serological responses to vaccination in prospectively recruited staff volunteers (appendix p 6). A detailed description of the methods, including the clinical cohort, virus culture conditions, genetic sequencing, and neutralisation assays, and the statistical analysis are available in the appendix (p 8). The Legacy study was approved by London Camden and Kings Cross Health Research Authority Research and Ethics committee (IRAS number 286469) and sponsored by University College London. Published in the Lancet (June 3, 2021):

|
Scooped by
Juan Lama
|
Background The SARS-CoV-2 pandemic has led to the development of various vaccines. Real-life data on immune responses elicited in the most vulnerable group of vaccinees over 80 years old is still underrepresented despite the prioritization of the elderly in vaccination campaigns. Methods We conducted a cohort study with two age groups, young vaccinees below the age of 60 and elderly vaccinees over the age of 80, to compare their antibody responses to the first and second dose of the BNT162b2 COVID-19 vaccination. Results While the majority of participants in both groups produced specific IgG antibody titers against SARS-CoV-2 spike protein, titers were significantly lower in elderly participants. Although the increment of antibody levels after the second immunization was higher in elderly participants, the absolute mean titer of this group remained lower than the <60 group. After the second vaccination, 31.3 % of the elderly had no detectable neutralizing antibodies in contrast to the younger group, in which only 2.2% had no detectable neutralizing antibodies. Conclusion Our data showed differences between the antibody responses raised after the first and second BNT162b2 vaccination, in particular lower frequencies of neutralizing antibodies in the elderly group. This suggests that this population needs to be closely monitored and may require earlier revaccination or/and an increased vaccine dose to ensure stronger long lasting immunity and protection against infection. Published in Clinical Infectious Diseases (April 27, 2021): https://doi.org/10.1093/cid/ciab381

|
Scooped by
Juan Lama
|
Vaccinating many people against SARS-CoV-2 could stall infection rates even among unvaccinated children in the same community. Last December, Israel launched one of the fastest vaccination schemes in the world, reaching 50% of the population in 9 weeks. But only people aged 16 and over were eligible for the jab. To test the ripple effects of widespread vaccination, Tal Patalon at Maccabi Healthcare Services in Tel Aviv-Yafo, Israel, Roy Kishony at the Technion — Israel Institute of Technology in Haifa and their colleagues analysed COVID-19 vaccinations and test results recorded between January and March 2021 for people in 223 Israeli communities (O. Milman et al. Preprint at medRxiv https://doi.org/f4d7; 2021). In each community, the authors examined the relationship between the vaccination rate in adults over three 3-week intervals and the rate of positive results for a COVID-19 test in children 35 days later. The authors found that, in the weeks after older people had received the Pfizer–BioNTech vaccine, the infection risk among children under 16 dropped proportionally to the percentage of adults who had been vaccinated. The authors warn that their results might be influenced by children who had previously been infected, even though the study included communities with low infection rates. The findings have not yet been peer reviewed. Preprint available in medRxiv (March 31, 2021): https://doi.org/10.1101/2021.03.26.21254394

|
Scooped by
Juan Lama
|
Clinical trial results of Pfizer/BioNTech's Covid-19 vaccine show it is 100% efficacious and well tolerated in youths 12-15. Pfizer/BioNTech plan to submit the data to the US Food and Drug Administration as soon as possible for expanded emergency use authorization of the two-dose vaccine. In a Phase 3 trial of 2,260 participants ages 12 to 15 in the US, the vaccine elicited strong antibody responses one month after the second dose -- exceeding those demonstrated in people ages 16 to 25 in previous trials, Pfizer reported. The vaccine is currently authorized in the US for emergency use in people 16 and older. Researchers observed 18 Covid-19 cases among the 1,129 participants who were given a placebo, and none among the 1,131 volunteers who got the vaccine. The data has yet to be peer reviewed. Pfizer/BioNTech added that the side effects seen in the young teens were similar to those seen among 16 to 25-year-olds. Common side effects include pain at the injection site, fatigue and fever. The participants will be monitored for protection and safety for two years after their second dose. Those comparisons to the older population are important, because researchers are building off of the knowledge they gained in the adult trials. Researchers can define a number of antibodies that are a correlate of the protection seen in adults, and then look for that level of antibodies in pediatric participants to know that the vaccine is providing protection. That's why the Covid-19 vaccine trials in children and adolescents have generally required fewer volunteers than the adult trials. "We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15," said Pfizer CEO Albert Bourla. "We plan to submit these data to FDA as a proposed amendment to our Emergency Use Authorization in the coming weeks and to other regulators around the world, with the hope of starting to vaccinate this age group before the start of the next school year." Dr. Peter Hotez, co-director of the Center for Vaccine Development at Texas Children's Hospital, said on CNN's New Day Wednesday, said schools can open without vaccinating students, but vaccines will help. "I think it's likely a green light to move forward, to move down in terms of vaccinating adolescents 12 to 15," Hotez said, noting that the vaccine will still need to be evaluated for authorization in that age group. "The bottom line is that by the fall I think there's a good possibility we'll be vaccinating teenagers, 12 and up, and for middle schools, junior high schools, high schools, it's really good news in the United States for both teachers and staff. We'll have teachers and staff vaccinated, we'll have the students vaccinated in those middle schools and high schools." A return to the classroom isn't the only factor at play. Health experts have emphasized the importance of protecting as many people as possible through vaccination, as more infectious Covid-19 variants continue to spread throughout the nation. "We all long for a normal life. This is especially true for our children," said BioNTech CEO Ugur Sahin. "The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the B.1.1.7 UK variant." Pfizer recently told CNN that the safety demonstrated in this adolescent trial helped the company make the decision to begin testing its vaccine in younger children. A separate Phase 1/2/3 study of the Pfizer/BioNTech vaccine in children ages 6 months to 11 years launched last week, when the first children ages 5 to 11 received a shot. Pfizer/BioNTech plans to begin dosing 2 to 5-year-olds next week and work its way down to participants ages 6 months to 2 years. The company aims to enroll 4,644 children in the trial and expects results by the end of 2021. Moderna is also testing its vaccine in adolescents and children, in two clinical trials of children ages 12 to 17 and those ages 6 months to 11 years. Experts anticipate Covid-19 vaccines won't be available for children 11 and younger in time for the upcoming school year. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, has said those younger children may have to wait until the first quarter of 2022. Dr. Buddy Creech, director of Vanderbilt University's Vaccine Research Program and an investigator in Moderna's pediatric trials, estimates a Covid-19 vaccine could be available for high-risk kids 12 and older by July or August, but likely won't be available for children 11 and younger until November or December, at the earliest.

|
Scooped by
Juan Lama
|
The COVID-19 vaccine from Pfizer Inc and BioNTech SE was able to neutralize a new variant of the coronavirus spreading rapidly in Brazil, according to a laboratory study published in the New England Journal of Medicine on Monday. Blood taken from people who had been given the vaccine neutralized an engineered version of the virus that contained the same mutations carried on the spike portion of the highly contagious P.1 variant first identified in Brazil, the study conducted by scientists from the companies and the University of Texas Medical Branch found. The scientists said the neutralizing ability was roughly equivalent the vaccine’s effect on a previous less contagious version of the virus from last year. The spike, used by the virus to enter human cells, is the primary target of many COVID-19 vaccines. In previously published studies, Pfizer had found that its vaccine neutralized other more contagious variants first identified in the United Kingdom and South Africa, although the South African variant may reduce protective antibodies elicited by the vaccine. Pfizer has said it believes its current vaccine is highly likely to still protect against the South African variant. However, the drugmaker is planning to test a third booster dose of their vaccine as well as a version retooled specifically to combat the variant in order to better understand the immune response. Original Study Published in N.Eng. J. Medicine (March 8, 2021): https://doi.org/10.1056/NEJMc2102017

|
Scooped by
Juan Lama
|
The researchers said the findings support policies delaying the second shot in efforts to stretch supplies and speed up immunizations. - The new study, conducted by Israel’s Sheba Medical Center, evaluated the effectiveness of the first shot of the Pfizer-BioNTech vaccine in around 7,000 healthcare workers.
- There was an 85% reduction in symptomatic Covid-19 cases within 15 and 28 days of receiving the shot, compared to an efficacy of around 95% for the manufacturer-recommended regime of two doses spaced 21 days apart.
- The single shot also helps prevent asymptomatic infections, the study found, with a 75% reduction in all infections detected after the single shot.
- The researchers said the findings support “delaying the second dose in countries facing vaccine shortages and scarce resources, so as to allow higher population coverage with a single dose.”
- Longer follow-up studies assessing the long-term effectiveness of using a single dose will be needed to inform any policies delaying the second dose, the researchers added.
The findings vindicate the U.K.’s controversial strategy of waiting 12 weeks between doses, which divided experts and irked manufacturers, who said the strategy exceeds the parameters of clinical trials used to test the vaccine. The approach was reluctantly endorsed by the World Health Organization, though it stopped short of the British approach with only a six week delay between doses recommended in “exceptional circumstances.” The U.S. has previously rejected proposals to stretch limited vaccine supplies by delaying the second dose, but officials are reportedly reconsidering the strategy amid rapidly spreading variants of the virus. Israel’s vaccination campaign is, by a significant margin, the world’s fastest. Nearly half of its population has been vaccinated with one shot and almost a third has been fully vaccinated with two. As officials begin to mull an exit from societal lockdown, Yuli Edelstein, the country’s health minister, described getting “getting vaccinated is a moral duty” and “part of our mutual responsibility.” Under proposed plans, those wishing to reenter society will likely have to carry a certificate of vaccination. Findigs in The Lancet (Feb. 18, 2021): https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00448-7/fulltext

|
Scooped by
Juan Lama
|
Background BNT162b2 vaccines showed high efficacy against COVID-19 in a randomized controlled phase-III trial. A vaccine effectiveness evaluation in real life settings is urgently needed, especially given the global disease surge. Hence, we assessed the short-term effectiveness of the first dose of BNT162b2-vaccine against SARS-CoV-2 infection. Given the BNT162b2 Phase-III results, we hypothesized that the cumulative incidence of SARS-CoV-2 infection among vaccinees will decline after 12 days following immunization compared to the incidence during the preceding days. Methods We conducted a retrospective cohort study using data from 2.6 million-member state-mandated health provider in Israel. Study population consisted of all members aged 16 or above years who were vaccinated with BNT162b2-vaccine between December/19/2020 and January/15/2021. We collected information regarding medical history and positive SARS-CoV-2 polymerase chain reaction test from days after first dose to January/17/2021. Daily and cumulative infection rates in days 13-24 were compared to days 1-12 after first dose using Kaplan-Meier survival analysis and generalized linear models. Findings Data of 503,875 individuals (mean age 59.7 years SD=14.7, 47.8% males) were analyzed, of whom 351,897 had 13-24 days of follow-up. The cumulative incidence of SARS-CoV-2 infection was 0.57% (n=2484) during days 1-12 and 0.27% (n=614) in days 13-24. A 51.4% relative risk reduction (RRR) was calculated in weighted-average daily incidence of SARS-CoV-2 infection from 43.41-per-100,000(SE=12.07) in days 1-12 to 21.08-per-100,000(SE=6.16) in days 13-24 following immunization. The decrement in incidence was evident from day 18 after first dose. Similar RRRs were calculated in individuals aged 60 or above (44.5%), younger individuals (50.2%), females (50.0%) and males (52.1%). Findings were similar in sub-populations and patients with various comorbidities. Conclusions We demonstrated an effectiveness of 51% of BNT162b2 vaccine against SARS-CoV-2 infection 13-24 days after immunization with the first dose. Immunization with the second dose should be continued to attain the anticipated protection.

|
Scooped by
Juan Lama
|
Pfizer Inc and BioNTech's COVID-19 vaccine appeared to work against a key mutation in the highly transmissible new variants of the coronavirus discovered in the UK and South Africa, according to a laboratory study conducted by the U.S. drugmaker. The not-yet peer reviewed study by Pfizer and scientists from the University of Texas Medical Branch indicated the vaccine was effective in neutralizing virus with the so-called N501Y mutation of the spike protein. The mutation could be responsible for greater transmissibility and there had been concern it could also make the virus escape antibody neutralization elicited by the vaccine, said Phil Dormitzer, one of Pfizer’s top viral vaccine scientists. The study was conducted on blood taken from people who had been given the vaccine. Its findings are limited, because it does not look at the full set of mutations found in either of the new variants of the rapidly spreading virus. Dormitzer said it was encouraging that the vaccine appears effective against the mutation, as well as 15 other mutations the company has previously tested against. “So we’ve now tested 16 different mutations, and none of them have really had any significant impact. That’s the good news,” he said. “That doesn’t mean that the 17th won’t.” Dormitzer noted another mutation found in the South African variant, called the E484K mutation, is also concerning. The researchers plan to run similar tests to see if the vaccine is effective against other mutations found in the UK and South African variants and hope to have more data within weeks. Scientists have expressed concern that vaccines being rolled out may not be able to protect against the new variants, particularly the one that emerged in South Africa. Simon Clarke, an associate professor in cellular microbiology at the University of Reading, said this week that while both variants had some new features in common, the one found in South Africa “has a number additional mutations” that included more extensive alterations to the spike protein. The Pfizer/BioNTech vaccine and the one from Moderna Inc, which use synthetic messenger RNA technology, can be quickly tweaked to address new mutations of a virus if necessary. Scientists have suggested the changes could be made in as little as six weeks. Findings available in bioRxiv (Jan. 7, 2021): https://www.biorxiv.org/content/10.1101/2021.01.07.425740v1
|



 Your new post is loading...
Your new post is loading...