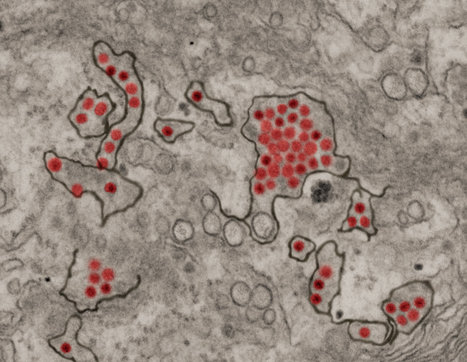
A massive upsurge in dengue cases marked by multiple outbreaks is occurring worldwide and raising new questions about who is at elevated risk of severe forms of the mosquito-transmitted disease. Incidence of the infection has increased by orders of magnitude throughout the so-called dengue belt, which encompasses Central and South America, Sub-Saharan Africa, Southeast Asia and swaths of the South Pacific, home to densely populated islands. Dengue, without question, is the most widespread and rapidly increasing vector-borne disease in the world, according to the World Health Organization. In the Americas alone, more than 5.2 million cases have been documented and more than 1,000 deaths were reported within the first three months of 2024, the Pan American Health Organization reported in April, noting a marked surge over the same period in 2023. The story is similar in other dengue-affected areas of the world where lapses in vector control have conspired with global climate change to create an explosion of bloodthirsty mosquitoes, swarms of them moving into regions once considered dengue-free. Only female mosquitoes feed on blood, they're in constant need of the nutrients in it to nurture their eggs. Now, more than two decades of dengue surveillance in Thailand is answering a slew of questions at a time when the world needs guidance most. Findings from the research have revealed how various subgroups—what virologists call subtypes—of the dengue virus influence future risk of severe infection. It has been known for years that those who become infected in subsequent outbreaks, after a usually mild bout with a first-time infection, are at significant risk of severe disease in later dengue exposures. New research finally has analyzed more than 15,000 cases to discern why that is so.
Writing in Science Translational Medicine, a global team of scientists has explained how the four dengue viral subtypes—DENV-1, 2, 3, and 4—influence the risk of repeated severe infections. The findings provide a new framework for disease monitoring and lay the foundation for vaccination strategies as the new dengue immunizations emerge. The team also underscored how dengue, a pernicious tropical malady, can be understood within the context of other common viral diseases that circle the globe. "The ability of viruses, such as SARS-CoV- 2 and influenza, to continuously change their genetic structure in response to the selective pressure of population immunity complicates control efforts," said Dr. Lin Wang, lead author of the dengue study. "In the case of dengue virus, an arbovirus that infects more than 100 million people each year, the situation is even more complex," Wang continued. "Individuals with high dengue virus antibody titers are protected from infection and developing severe disease. "However, individuals with sub-neutralizing antibody titers have been shown to have the highest risk of severe disease, through multiple hypothesized mechanisms including antibody-dependent enhancement," emphasized Wang, a researcher in the genetics department at the University of Cambridge in England. A dengue infection can be tricky. Some patients who have weathered an infection but get infected in a subsequent outbreak can have more severe symptoms the second time around. Yet, most research on repeat dengue infections has regarded each of the serotypes as no different from the other, Wang and colleagues contend, noting that an assessment of each serotype's genetic differences was needed to provide a clearer picture of potential risks.
To develop that clearer picture, researchers studied each serotype in more than 15,000 patients' infections as a way to peel away much of the mystery surrounding why first-time dengue illnesses are traditionally milder than subsequent ones. Working with Wang were collaborators from two centers in Bangkok, Thailand; multiple research institutes in the United States and one in France. To determine how each of the viral serotypes affects the risk of severe disease, Wang and colleagues analyzed viral genetic data. The team also studied cases of patients hospitalized for dengue to determine which viral subtype caused their infections. Researchers gathered data from 21 years of dengue surveillance, ranging from 1994 to 2014, in a children's hospital in Bangkok, encompassing 15,281 individual cases. This allowed them to find repeat cases and each viral subtype in all infections. Based on the pediatric patients' hospital records, researchers discovered a link between hospitalization and the order in which patients became infected with different dengue-virus serotypes. They were also able to determine which combinations of viral subtypes pointed to mild or severe forms of dengue. For instance, people who became infected with serotypes that were very similar, such as DENV-3 and DENV-4, or very different serotypes as in the case of DENV-1 and DENV-4, tended to have a lower risk of severe disease during the second infection. That said, patients who were infected with serotypes that were only moderately different had a higher risk of severe symptoms in subsequent infections. The highest risk group in this category involved patients who had an initial infection with DENV-2 followed by a subsequent infection triggered by DENV-1.
The new research adds clarity to a disease risk that may seem paradoxical to the lay public. For example, most people infected with dengue virus for the first time develop extremely mild signs of the disease or none at all. But for those who do get sick, soaring fever, headache, body aches, nausea and rash are the primary symptoms, and they intensify in severe manifestations of the infection. For more than a century a severe bout with dengue has been known as breakbone fever because of the intensity of the pain and accompanying muscle spasms. The virus is carried in the tropics and subtropics by Aedes aegypti and Aedes albopictus mosquitoes, which are endemic in the dengue belt. But while the belt, which runs through latitudes 35-degrees North and 35-degrees South, has traditionally been home to dengue-carrying mosquitoes, the arthropods have been extending their range northward as global climate change intensifies, scientists say. Wang, meanwhile, reports that the collaborative research has set the stage to better understand immune system function in subsequent, severe dengue infections. "These findings suggest that immune imprinting helps determine dengue disease risk and provides a pathway to monitor the changing risk profile of populations and to quantifying risk profiles of candidate vaccines," Wang concluded. "This will become increasingly important as dengue vaccines begin to get used."
Research published in Science Translational Medicine (April 24, 2024):
https://doi.org/10.1126/scitranslmed.adk3259



 Your new post is loading...
Your new post is loading...