 Your new post is loading...

|
Scooped by
Juan Lama
|
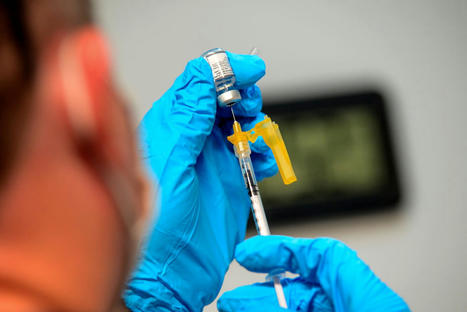
Most people are still not eligible for a second booster, but the FDA will revisit the topic after an expert panel meets in June. The Food and Drug Administration on Tuesday approved a second omicron booster from Moderna and Pfizer for people over the age of 65 and immunocompromised individuals. Why it matters: An additional dose of the bivalent vaccine can help high-risk individuals with waning immunity to COVID, the FDA said. - Most people who have received a single shot of the bivalent vaccine aren't eligible for a second dose, but the FDA could revise criteria after an expert panel meets to discuss the topic in June.
Details: Tuesday's decision means those over 65 can get a second booster no sooner than four months after the first. - Immunocompromised people may receive the second shot at least two months after receiving the first dose "and additional doses may be administered at the discretion of, and at intervals determined by, their health care provider," the FDA said.
- The agency made additional changes, saying that unvaccinated people may receive a single dose of the bivalent vaccine as their first shot, rather than multiple doses of the original monovalent vaccine.
- Children between six months and five years old who have received up to three doses of the monovalent vaccine may receive a bivalent vaccine shot, but the number of doses they receive "will depend on the vaccine and their vaccination history," per the FDA.
- The monovalent shots from Moderna and Pfizer are no longer authorized for use in the U.S.
What they're saying: "At this stage of the pandemic, data support simplifying the use of the authorized mRNA bivalent COVID-19 vaccines and the agency believes that this approach will help encourage future vaccination," said Peter Marks, director of FDA's Center for Biologics and Research. - "Evidence is now available that most of the U.S. population 5 years of age and older has antibodies to SARS-CoV-2 ... either from vaccination or infection that can serve as a foundation for the protection provided by the bivalent vaccines," Marks added.
Catch up fast: An FDA advisory committee in January unanimously recommended that the U.S. replace initial doses of the original COVID monovalent shots with bivalent ones to target omicron subvariants, directing Pfizer, Moderna and Novavax to focus on bivalent vaccines. - At the time, the FDA requested that the panel consider a future immunization schedule and proposed a yearly one-dose schedule for the general population and two doses for high-risk individuals.
- The panel, however, did not make any specific recommendations on that because members said they needed additional data on different population groups to determine what dosage was appropriate for each group.
What we're watching: A panel for the Centers for Disease Control and Prevention is scheduled to meet on Wednesday to discuss the second booster strategy. - If panel recommends the shot for high-risk individuals and CDC Director Rochelle Walensky signs off, the boosters could be available this week.
Zoom out: The FDA's decision comes as a new subvariant, XBB. 1.16, known as Arcturus, has been spreading around the world and gaining traction in the U.S. Don't forget: The COVID public health emergency is set to end in less than a month, shifting the cost of vaccines and other countermeasures to the private market. FDA Press Release (April 18, 2023): https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-changes-simplify-use-bivalent-mrna-covid-19-vaccines

|
Scooped by
Juan Lama
|
WASHINGTON — The Food and Drug Administration on Wednesday authorized the first redesign of coronavirus vaccines since they were rolled out in late 2020, setting up millions of Americans to receive new booster doses targeting Omicron subvariants as soon as next week. The agency cleared two options aimed at the BA.5 variant of Omicron that is now dominant: one made by Pfizer and its German partner BioNTech for use in people as young as 12, and the other by Moderna, for those 18 and older. The doses can be given at least two months since people last received a booster dose or completed their initial series of vaccinations. Biden administration officials have argued that even as researchers work to understand how protective the new shots might be, inoculating Americans again in the coming weeks could help curb the persistently high number of infections and deaths. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose,” Dr. Robert M. Califf, the F.D.A. commissioner, said in a statement on Wednesday. He added that the vaccine would “provide better protection against currently circulating variants.” An average of about 90,000 infections and 475 deaths are recorded every day around the United States, almost three years into a pandemic that has killed more than a million Americans and driven a historic drop in life expectancy. But there are also hopeful signs. Even with high case counts, fewer than 40,000 people are currently hospitalized with the virus, a decrease of 10 percent since early August and far fewer than during the Delta-driven surge last summer or the Omicron-fueled wave last winter. Deaths have also remained somewhat flat in recent weeks, a sign that vaccines are helping to prevent the worst outcomes of Covid-19. Ample evidence suggests that many Americans will hold back from getting the updated boosters, either because they are weary of the pandemic or may not feel urgency about an additional dose. With each new shot offered, there are fewer takers. As more companies bring workers back to offices and students return to campuses this fall, persuading Americans to get the updated booster shots will be a major challenge for the administration. The companies produced the retooled shots with extraordinary speed, a testament to the mRNA technology that Pfizer and Moderna have harnessed since the early months of the coronavirus outbreak. The Food and Drug Administration advised companies only two months ago on the formulation that they should adopt for the new vaccines. By later this week, millions of those doses will be delivered to states. The tight timeline meant that the companies went to federal regulators this summer with more limited data on the redesigned boosters than a traditional review process would call for, generating some controversy. Regulators authorized the vaccine without results from human trials, which have just started. Federal officials argue that because the coronavirus is evolving so quickly, human trials would be out of date before they can deliver results that could be used in the F.D.A.’s authorization decision. Instead, they are relying on the results of mouse trials and earlier human trials by Pfizer and Moderna of reformulations aimed at previous versions of the virus. The Biden administration is casting the shots as a standard upgrade that Americans should embrace ahead of potential surges in cases in the winter, like the flu shot, which is reconfigured every year to target more current versions of the influenza virus. The boosters are arriving during a period when the White House has been largely quiet on the pandemic. President Biden has rarely commented on the coronavirus in recent months, even after he tested positive in July. The White House no longer holds regular news briefings on the federal pandemic response, as it did in the first year of the administration — a reflection of the weariness of many Americans in keeping up with Covid precautions. “Covid-19 is the third leading cause of death in the United States. And it’s as if we’ve just accepted that that is going to be the case,” said Mercedes Carnethon, an epidemiologist at Northwestern University’s Feinberg School of Medicine. “I really hope as many people as possible will seek the updated booster so we can protect those who will have a terrible outcome.” Vaccinations remain the cornerstone of the federal government’s Covid strategy, even with tests and treatments widely available. The Biden administration has ordered over 170 million doses for the fall campaign, and officials do not expect shortages when they are rolled out. “If it’s freezing cold out and you have children, you’re going to dress them warmly. This is the concept here,” said Dr. Paul G. Auwaerter, the clinical director of the infectious diseases division at the Johns Hopkins University School of Medicine. “You’ll want to head into the respiratory season with a virus that we know has surprised us with a booster.” Exactly how protective the shots might be is unknown, Dr. Auwaerter said. He pointed to the modest increases in neutralizing antibodies that the companies found in vaccines they tested this year that targeted the original form of Omicron. How antibody levels would translate to protection with the new vaccines was unclear, he added. Experts warned against trying to choose Moderna’s shot over Pfizer’s or vice versa; with research in humans just beginning, scientists are months from knowing whether one brand offers better protection than the other. Many Americans have recently been infected with variants in the Omicron family and have some protection from their bouts with the virus, a development that federal agencies may take into account when recommending how the new shots are used. An advisory committee to the Centers for Disease Control and Prevention is scheduled to meet this week to make recommendations. “For most people, the risk of death is so low at this point, because they’ve gotten infected or vaccinated, or more likely both,” said Dr. Gregory A. Poland, a professor of medicine and infectious diseases and the director of the Vaccine Research Group at the Mayo Clinic. Dr. Poland, who has advised Moderna, Pfizer and White House officials on coronavirus vaccines, said updating booster shots the way the Food and Drug Administration did on Wednesday amounted to a “chase your tail” strategy, tweaking the design incrementally to try to keep up with a fast-changing virus. The new boosters, he said, could potentially save some lives among the elderly and those with immune deficiencies. But they were unlikely to make as substantial an impact with the rest of the population.
|

|
Scooped by
Juan Lama
|
The Biden administration is moving to make the shot available for older people and those with impaired immune systems. While federal authorities are poised to authorize a second dose of the omicron-targeting coronavirus booster for vulnerable groups, not all experts believe it is necessary. (Kristopher Radder/Brattleboro Reformer/AP) Federal regulators have decided to authorize a second omicron-specific coronavirus vaccine booster shot for people who are at least 65 or have weak immune systems — an effort to provide additional protection to high-risk individuals, according to several officials familiar with the plan. The Food and Drug Administration is expected to announce the step in the next few weeks, and the Centers for Disease Control and Prevention is expected to move quickly to endorse it, said the officials, who spoke on the condition of anonymity because they were not authorized to publicly discuss internal discussions. Eligible individuals will be able to receive the dose as long as it has been at least four months since their first shot of what’s known as the bivalent booster, which targets omicron subvariants BA.4 and BA.5 as well as the original novel coronavirus. The expectation is that consumers will consult with their health-care providers about whether to get the extra booster, the officials said. The FDA’s policy change will be “permissive” — people may get the shot but will not be told they should get it, the officials said. It’s not clear whether the CDC’s vaccine advisory committee would meet to discuss the change before CDC Director Rochelle Walensky makes a final recommendation. Doctors and other experts have expressed mixed views about a second bivalent booster. Some say there is little data to justify it, while others argue it would benefit high-risk individuals who received their first omicron-targeting shot last fall and probably have reduced protection as the effects fade. Some anxious patients have been “really clamoring” for a second omicron booster, said Camille Kotton, clinical director for transplant and immunocompromised host infectious diseases at Massachusetts General Hospital in Boston. At a recent meeting of the CDC vaccine advisory panel, she said she would support allowing additional booster doses for high-risk groups, especially for the most significantly immunocompromised patients. Jamie Loehr, a family medicine doctor in Ithaca, N.Y., who is also a member of the vaccine advisory panel, said there is evidence that older people and those with weak immune systems don’t produce especially robust responses to vaccines in general, and to the coronavirus vaccine in particular. It would seem reasonable to give them more frequent boosters, he wrote in an email, but he wants to see data before deciding whether he would support a more frequent booster for these groups. John P. Moore, a professor of microbiology and immunology at Weill Cornell Medicine in New York, said an extra booster could benefit people who are in poor health or have an impaired immune system. But he was skeptical everyone older than 65 needs it. Boosters lead to “a short-term boost against mild infection but protection against severe disease is still pretty robust” because of previous shots, he said. Moore said it is a mistake to think “everyone over a certain age is in the same health bracket,” when in fact health status varies widely. He said he is older than 65 and healthy — and “not giving a moment’s thought about getting another booster, though I might next winter if infections tick up.” Administration officials acknowledge there is not extensive data on the bivalent vaccine, which was first authorized in August. But they said real-world data and smaller studies are consistent with large studies on the original vaccine showing that its protection against symptomatic infection fades after several months. In addition, unpublished data presented at the CDC’s vaccine advisory panel meeting in February confirmed earlier real-world reports that bivalent vaccines are providing protection against serious illness — emergency room visits and hospitalizations — in adults, compared with people who received previous doses of the original vaccine and no omicron-targeting dose. Other studies also suggest older people might be better shielded from serious illness with an additional booster, the officials said. They noted they are not advocating the second omicron booster for young people, who might experience rare heart-related side effects. The Wall Street Journal and NPR previously reported that the FDA was considering a second bivalent booster for high-risk groups. Only about 42 percent of people 65 and older have received the first bivalent booster dose, according to the CDC. A second booster will not affect the FDA’s plan to move to a once-a-year coronavirus vaccine booster for most Americans — a strategy announcedby the agency in January, the officials said. This summer, the agency and its advisers will select a retooled formula for an updated vaccine to be deployed during a campaign planned for next fall. The formula will be based on which coronavirus strain scientists think will be circulating in the fall and winter. In a statement, the FDA said: “We continue to closely monitor the emerging data in the United States and globally, and we will base any decision on additional updated boosters upon those data. Importantly, individuals who have not yet received an updated (bivalent) booster are encouraged to speak with their health-care provider and consider receiving one.” The updated shot will be free, regardless of insurance coverage, because the government has an ample supply of boosters. Even after the federal supply of vaccines is gone, shots will continue to be free to most people with private and public health insurance. But once federally purchased doses are depleted, uninsured and underinsured adults may have to pay, and privately insured people might need to confirm that their provider is in-network, according to an analysis by the Kaiser Family Foundation. Peter Hotez, dean of the National School of Tropical Medicine and professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine in Houston, said he is a strong proponent of making a second omicron-targeting booster available for everyone 50 and older. Michael T. Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, agreed, saying a second booster would provide an additional tool for people who have been conscientious about reducing their risks of contracting covid. An omicron subvariant, XBB.1.5, now accounts for almost 90 percent of the cases in the United States, according to the CDC.
|



 Your new post is loading...
Your new post is loading...









buy Xanax Alprazolam
Price: $165.0 – $417.0Buy Xanax Online without prescription
Price: $720.0 $247.0Buy yellow xanax bars online
Price: $200.0 – $600.0Buy Zopiclone online uk
Price: $200.0 – $650.0Endocet for sale online
Price: $180.0 – $450.0Order Acxion Fentermina 30mg
Price: $200.0 – $600.0oxycontin for sale online
Price: $200.0 – $1,000.0phentermine for sale online
tramadol for sale online
Price: $100.0 – $900.0Where to buy Ecstasy Online